Abstract
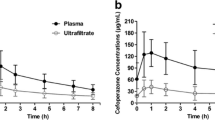
This study examined the pharmacokinetics of arbekacin during continuous venovenous hemodiafiltration (CVVHDF) and assessed the pharmacodynamics to consider arbekacin dosage adaptation in CVVHDF. Arbekacin was administered by 0.5-h infusion once daily, using a polymethyl methacrylate membrane hemofilter, to three critically ill patients undergoing CVVHDF; the flow rates were 0.8 l/h for the filtrate and 0.6 l/h for the dialysate. The drug concentrations in plasma and in the filtrate-dialysate were determined using a fluorescence polarization immunoassay and analyzed pharmacokinetically. The average sieving coefficient of arbekacin was 0.739 and the average drug clearance by CVVHDF was 1.03 l/h. A pharmacokinetic model with three compartments (1, central; 2, peripheral; 3, filtrate-dialysate side hemofilter) accurately reflected the concentration-time data for both plasma and filtrate-dialysate. The pharmacokinetic model assessed the pharmacodynamic profile of arbekacin once-daily regimens (0.5-h infusions) at filtrate-dialysate flow rates of 1.4 and 2.8 l/h, and demonstrated that only the 150-mg and 200-mg regimens achieved an effective target range for Cmax (9–20 µg/ml), suggesting that empirical dosages lower than the usual 150–200 mg should be avoided in patients undergoing CVVHDF. The minimum regimens needed to achieve an effective pharmacodynamic target for the free Cmax/MIC ratio (>8) were 75 mg for an MIC of 0.5 µg/ml, 200 mg for an MIC of 2 µg/ml, and 400 mg for an MIC of 4 µg/ml. These results will help us to better understand the pharmacokinetics of arbekacin during CVVHDF, while also helping in the selection of the appropriate arbekacin regimens, based on a pharmacodynamic assessment, for patients receiving this renal replacement therapy.
Similar content being viewed by others
References
Oka T, Tanaka T, Omori Y, Ishimine G, Ueda Y, Shimizu M, et al. Fundamental and clinical study on HBK, a new aminoglycoside in surgical field. Jpn J Chemother 1986;34(Suppl 1):575–582.
Fillastre JP, Leroy A, Humbert G, Moulin B, Bernadet P, Josse S. Pharmacokinetics of habekacin in patients with renal insufficiency. Antimicrob Agents Chemother 1987;31:575–577.
Arakawa S, Maeda H, Fujii A, Kamidono S, Hamada K, Miyazaki S, et al. Clinical study of aminoglycosides on renal dysfunction. Hinyokika Kiyo (Acta Urologica Japonica) 1989;35:697–704.
Matsuo H, Hayashi J, Ono K, Andoh K, Andoh Y, Sano Y, et al. Administration of aminoglycosides to hemodialysis patients immediately before dialysis: a new dosing modality. Antimicrob Agents Chemother 1997;41:2597–2601.
Kaneoka Y, Hyodo T, Kondo M, Akaishi H, Honma T, Yamamoto S, et al. The removal rates of vancomycin and arbekacin by various kinds of dialyzers. Jpn J Urol Surg 2005;18:817–823.
Okada K, Suzuki T, Nakamura H, Ishikawa K, Ariyoshi N, Nakazawa K, et al. Medication design of arbekacin based on hemodialysis schedule. Jpn J Ther Drug Monit 2005;22:27–33.
Yamaoka K, Tanigawara Y, Nakagawa T, Uno T. A pharmacokinetic analysis program (multi) for microcomputer. J Pharmacobiodyn 1981;4:879–885.
Robatel C, Decosterd LA, Biollaz J, Eckert P, Schaller MD, Buclin T. Pharmacokinetics and dosage adaptation of meropenem during continuous venovenous hemodiafiltration in critically ill patients. J Clin Pharmacol 2003;43:1329–1340.
Giles LJ, Jennings AC, Thomson AH, Creed G, Beale RJ, McLuckie A. Pharmacokinetics of meropenem in intensive care unit patients receiving continuous veno-venous hemofiltration or hemodiafiltration. Crit Care Med 2000;28:632–637.
Ikawa K, Morikawa N, Ikeda K, Suyama H. Pharmacokinetic modeling and dosage adaptation of biapenem in Japanese patients during continuous venovenous hemodiafiltration. J Infect Chemother 2008;14:35–39.
Mitomi N, Matsumoto T, Fujigaki M, Komiya I, Kai F. Absorption, distribution, and excretion of arbekacin after intravenous and intramuscular administration in rats. Jpn J Antibiot 1987;40:357–364.
Tanikaze N, Komatsu M, Shimakawa K, Yamamoto I. Study of clinical significance of PK/PD (pharmacokinetic/pharmacodynamic) parameters after administering arbekacin to patients with pulmonary methicillin-resistant Staphylococcus aureus infection. Jpn J Chemother 2004;52:469–473.
Mikuniya T, Kato Y, Muto-Kobayashi Y, Sanbongi Y, Shimizu A, Hiraishi T, et al. Prevalence of drug resistant gene and changes in susceptibility of methicillin-resistant Staphylococcus aureus strains isolated from 1990 to 2006 in Japan to antimicrobial agents. Jpn J Chemother 2009;57:37–40.
Scaglione F. Can PK/PD be used in everyday clinical practice? Int J Antimicrob Agents 2002;19:349–353.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ikawa, K., Morikawa, N., Suyama, H. et al. Pharmacokinetics and pharmacodynamics of once-daily arbekacin during continuous venovenous hemodiafiltration in critically ill patients. J Infect Chemother 15, 420–423 (2009). https://doi.org/10.1007/s10156-009-0717-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10156-009-0717-5