Abstract
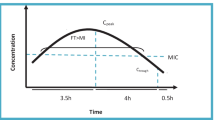
New recommendations for vancomycin (VCM) dosages and dosing intervals for MRSA-infected pneumonia patients with various degrees of renal function impairment were established based on Japanese population pharmacokinetic parameters proposed by Yasuhara et al. in 1998. Based on individual creatinine clearance (CLcr), we proposed the optimum VCM dosages and dosing intervals so that the peak level of VCM (level at 1 h after the end of infusion) is maintained in the range of 25–40 µg/ml and the trough level is kept under 15 µg/ml. The recommended doses and intervals of VCM were as follows: 20 mg/kg every 12 h for CLcr of 80–100 ml/min, 18 mg/kg every 12 h for CLcr of 70 ml/min, 25 mg/kg every 24 h for CLcr of 50–60 ml/min, 22 mg/kg every 36 h for CLcr of 40 ml/min, and 18 mg/kg every 48 h for CLcr of 30 ml/min. Using the recorded pharmacokinetic parameters of VCM from eight patients with pneumonia who were admitted to Aoyama Second Hospital between November 1997 and January 2002, these recommendations were used in computer simulations for the eight patients, and the usefulness of these recommendations was confirmed.
Similar content being viewed by others
References
TC Sorrell DR Packham S Shanker M Foldes R Munro (1982) ArticleTitleVancomycin therapy for methicillin-resistant Staphylococcus aureus Ann Intern Med 97 344–50 Occurrence Handle7114631
RA Blum KA Rodvold (1987) ArticleTitleRecognition and importance of Staphylococcus epidermidis infections Clin Pharm 6 464–75 Occurrence Handle3319361
RC Moellering DJ Krogstad DJ Greenblatt (1981) ArticleTitleVancomycin therapy in patients with impaired renal function: a nomogram for dosage Ann Intern Med 94 343–6 Occurrence Handle6101256
KA Rodvold RA Blum JH Fischer HZ Zokufa JC Rotshafer KB Crossley et al. (1988) ArticleTitleVancomycin pharmacokinetics in patients with various degrees of renal function Antimicrob Agents Chemother 32 848–52 Occurrence Handle3415206
GR Matzke RW McGory CE Halstenson WF Keane (1984) ArticleTitlePharmacokinetics of vancomycin in patients with various degrees of renal function Antimicrob Agents Chemother 25 433–7 Occurrence Handle6732213
KA Rodvold RD Pryka M Garrison JC Rotschafer (1989) ArticleTitleEvaluation of a two-compartment Bayesian forecasting program for predicting vancomycin concentrations Ther Drug Monit 11 269–75 Occurrence Handle2728085
M Yasuhara T Iga H Zenda K Okumura T Oguma Y Yano et al. (1998) ArticleTitlePopulation pharmacokinetics of vancomycin in Japanese adult patients Ther Drug Monit 20 139–48 Occurrence Handle10.1097/00007691-199804000-00003 Occurrence Handle9558127
M Cruciani G Gatti L Lazzarini G Furlan G Broccali M Malena et al. (1996) ArticleTitlePenetration of vancomycin into human lung tissue J Antimicrob Chemother 38 865–9 Occurrence Handle8961057
K Niitsuma M Saito (1996) ArticleTitleVCM inhalation therapy Antibiot Chemother 12 123–35
Rybak MJ, Cappelletty DM, Ruffing MJ, et al. Influence of vancomycin serum concentrations on the outcome of patients being treated for gram-positive infections. Abstract 37th Intersci Conference. Antimicrob Agents Chemother 1997;A46.
Y Yano T Oguma (1997) ArticleTitleA pharmacokinetics analysis software for TDM based on the Bayesian estimation using visual basic Jpn J TDM 14 179–88
GR Matzke GG Zhanel DR Guay (1986) ArticleTitleClinical pharmacokinetics of vancomycin Clin Pharmacokinet 11 257–82 Occurrence Handle3530582
GR Matzke (1992) Vancomycin WE Evans JJ Shentag WJ Jusko (Eds) Applied pharmacokinetics EditionNumber3rd ed. Applied Therapeutics Vancouver 15.2–15.31
AP MacGrowan (1998) ArticleTitlePharmacodynamics, pharmacokinetics, and therapeutic drug monitoring of glycopeptides Drug Monit 20 473–7 Occurrence Handle10.1097/00007691-199810000-00005
S Shalansky (1995) ArticleTitleRationalization of vancomycin serum concentration monitoring Can J Hosp Pharm 48 17–24
JM Hyatt PS Mckinnon GS Zimmer JJ Schentag (1995) ArticleTitleThe importance of pharmacokinetic/pharmacodynamic surrogate markersto outcome. Focus on antibacterial agents Clin Pharmacokinet 28 143–60 Occurrence Handle7736689
WA Craig (2003) ArticleTitleBasic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid Infect Dis Clin North Am 17 479–501 Occurrence Handle10.1016/S0891-5520(03)00065-5 Occurrence Handle14711073
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Yoshida, M., Yasuda, N., Nishikata, M. et al. New recommendations for vancomycin dosage for patients with MRSA pneumonia with various degrees of renal function impairment. J Infect Chemother 11, 182–188 (2005). https://doi.org/10.1007/s10156-005-0394-y
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10156-005-0394-y