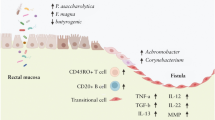
Abstract
Background
Since our last publication of algorithms for the management of perianal fistulas in patients with Crohn’s disease, researchers have proposed a treat to target strategy systematic combotherapy for anal lesions, and indications for stem cell injection. In the absence robust publications, the Société Nationale Française de Coloproctologie (French National Society of Coloproctology [SNFCP]) wished to establish a group consensus using the Delphi method.
Methods
From October 2020 to January 2021, a scientific committee and panel of gastroenterologists and surgeons established answers which were submitted to the members of the SNFCP during a national conference in November 2020. Three questions were clarified and reformulated, and then submitted during a third and final round of consultation of members of the SNFCP.
Results
The target was defined as being the response obtained in every domain (symptoms, physical and radiological evaluation) which could be considered satisfactory, without the need to intensify therapeutic management. By consensus, the time required for clinical evaluation of the efficacy of treatment was 6 months. A response on magnetic resonance imaging (MRI) should include the absence of a collection of 10 mm or more in size at 6 months, and a frank decrease or complete disappearance of hyperintensity in T1 and T2 sequences of the main tract at 12 months. Systematic association of an immunosuppressant with tumor necrosis factor inhibitors did not reach the consensus level for adalimumab (50%), but just did for infliximab (70%). The majority of the respondents considered failure of one, or even two lines of different biotherapies to be potential indications for injection of stem cells.
Conclusions
These findings reinforce the importance of composite targets including MRI evaluation, and underscore the need for precise timing of evaluation. Combotherapy is only recommended with infliximab. Injection of stem cells is a second- or third-line option.





Similar content being viewed by others
References
Bouchard D, Pigot F, Staumont G, Siproudhis L, Abramowitz L, Benfredj P et al (2018) Management of anoperineal lesions in Crohn’s disease: a French National Society of Coloproctology national consensus. Tech Coloproctol 22:905–917
Hommes D, Colombel JF, Emery P, Greco M, Sandborn WJ (2012) Changing Crohn’s disease management: need for new goals and indices to prevent disability and improve quality of life. J Crohns Colitis 6(Suppl2):S224–S234
Irvine EJ (1995) Usual therapy improves perianal Crohn’s disease as measured by a new disease activity index McMaster IBD Study Group. J Clin Gastroenterol 20:27–32
Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, van Hogezand RA et al (1999) Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med 340:1398–1405
Panés J, García-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC et al (2016) Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised double-blind controlled trial. Lancet 388:1281–1290
Lichtenstein GR, Diamond RH, Wagner CL, Fasanmade AA, Olson AD, Marano CW et al (2009) Benefits and risks of immunomodulators and maintenance infliximab for IBD-subgroup analyses across four randomized trials. Alimentary Pharmacol Therap 30:210–226
Bouguen G, Siproudhis L, Gizard E, Wallenhorst T, Billioud V, Bretagne JF et al (2013) Long-term outcome of perianal fistulizing Crohn’s disease treated with infliximab. Clin Gastroenterol Hepatol 11:975–981
Kopylov U, Al-Taweel T, Yaghoobi M, Nauche B, Bitton A, Lakatos PL et al (2014) Adalimumab monotherapy versus combination therapy with immunomodulators in patients with Crohn’s disease: a systematic review and meta-analysis. J Crohns Colitis 8:1632–1641
Colombel JF, Jharap B, Sandborn WJ, Feagan B, Peyrin-Biroulet L, Eichner SF et al (2017) Effects of concomitant immunomodulators on the pharmacokinetics, efficacy and safety of adalimumab in patients with Crohn’s disease or ulcerative colitis who had failed conventional therapy. Aliment Pharmacol Ther 45:50–62
Molendijk I, Bonsing BA, Roelofs H, Peeters KC, Wasser MN, Dijkstra G et al (2015) Allogeneic bone marrow-derived mesenchymal stromal cells promote healing of refractory perianal fistulas in patients with Crohn’s disease. Gastroenterology 149:918–927
Levesque BG, Sandborn WJ, Ruel J, Feagan BG, Sands BE, Colombel JF (2015) Converging goals of treatment of inflammatory bowel disease from clinical trials and practice. Gastroenterology 148:37–51
Sahnan K, Tozer PJ, Adegbola SO, Lee MJ, Heywood N, McNair AGK et al (2019) Developing a core outcome set for fistulising perianal Crohn’s disease. Gut 68:226–238
Malian A, Rivière P, Bouchard D, Pigot F, Eléouet-Kaplan M, Favreau-Weltzer C et al (2020) Predictors of perianal fistula relapse in Crohn’s disease. Inflamm Bowel Dis 26:926–931
Kennedy NA, Heap GA, Green HD, Hamilton B, Bewshea C, Walker GJ et al (2019) UK Inflammatory Bowel Disease Pharmacogenetics Study Group. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol 4:341–353
Davidov Y, Ungar B, Bar-Yoseph H, Carter D, Haj-Natour O, Yavzori M et al (2017) Association of induction infliximab levels with clinical response in perianal Crohn’s disease. J Crohns Colitis 11:549–555
Yarur AJ, Kanagala V, Stein DJ, Czul F, Quintero MA, Agrawal D et al (2017) Higher infliximab trough levels are associated with perianal fistula healing in patients with Crohn’s disease. Aliment Pharmacol Ther 45:933–940
Acknowledgements
The members of the SNFCP and Members of the GETAID: Guillaume Bouguen (Digestive Diseases Unit, CHU Pontchaillou, 2 rue Henri Le Guilloux 35033 Rennes, France), David Laharie (Hepato-Gastroenterology Unit, CHU Haut Lévèque, 33600 Pessac ), Eddy Cotte (Digestive and Endocrine Surgery Unit, Hospital Lyon-Sud, 165 chemin du grand Revoyet, 69495 Pierre Bénite cedex ), Yves Panis (Digestive Surgery Unit, Beaujon Hospital, 100 boulevard du Général Leclerc, 92110 Clichy), L Peyrin-Biroulet (Hepato-Gastroenterology Unit, CHRU Nancy, 54511 Vandoeuvre les Nancy), Xavier Roblin (Gastroenterology, Hepatology and Inflammatory Bowel Diseases Unit, CHU Saint Etienne, 25 boulevard Pasteur, 42055 Saint-Etienne), Philippe Zerbib (Digestive Unit, Hospital Huriez, rue Michel Polonowski, 59037 Lille) as co-authors, Emmanuelle Babin-Pigot and Marie Ferry for essential logistic assistance.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
DB: Takeda as consultant, Abbvie Janssen Takeda for learning. LS: Takeda Abbvie Janssen MSD for clinical research, Takeda as consultant, Takeda Abbvie Janssen Amgen Ferring for learning. VdP: Takeda as consultant, Abbvie, Amgen, Tillots for learning. GS: Takeda, Janssen as consultant, Abbvie, Takeda for learning. LA: Abbvie, Takeda, Celltrion for clinical research, Takeda as consultant, Takeda, Abbvie, Janssen, Ferring for learning. FP: Abbvie, Takeda for learning.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The Members of SNFCP and Members of GETAID were listed in Acknowledgement section.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bouchard, D., Pigot, F., de Parades, V. et al. Management of perianal fistulas in Crohn’s disease: a 2021 update of the French National Society of Coloproctology consensus. Tech Coloproctol 26, 805–811 (2022). https://doi.org/10.1007/s10151-022-02678-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-022-02678-x