Abstract
Background
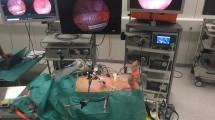
Recognition of a non-viable bowel during colorectal surgery is a challenging task for surgeons. Identifying the turning point in serosal microcirculatory deterioration leading up to a non-viable bowel is crucial. The aim of the present study was to determine whether sidestream darkfield (SDF) imaging can detect subtle changes in serosal microcirculation of the sigmoid after vascular transection during colorectal surgery.
Methods
A prospective observational clinical study was performed at a single medical centre. All eligible participants underwent laparoscopic sigmoid resection and measurements were taken during the extra-abdominal phase. Microcirculation was measured at the transected bowel and 20 cm proximal to this point. Microcirculatory parameters such as Microvascular Flow Index (MFI), proportion of perfused vessels (PPV), perfused vessel density (PVD), total vessel density (TVD) and the Heterogeneity Index were determined. Data are presented as median (interquartile range) or mean ± standard deviation.
Results
A total of 60 SDF images were acquired for 10 patients. Perfusion parameters and perfused vessel density were significantly lower at the transected bowel compared with the non-transected measurements [MFI 2.29 (1.96–2.63) vs 2.96 (2.73–3.00), p = 0.007; PPV 74% (55–83) vs 94% (86–97), p = 0.007; and PVD 7.61 ± 2.99 mm/mm2 versus 10.67 ± 1.48 mm/mm2, p = 0.009]. Total vessel density was similar between the measurement locations.
Conclusions
SDF imaging can identify changes of the bowel serosal microcirculation. Significantly lower serosal microcirculatory parameters of the vascular transected bowel was seen compared with the non-transected bowel. The ability of SDF imaging to detect subtle differences holds promise for future research on microvascular cut-off values leading to a non-viable bowel.



Similar content being viewed by others
References
Attard JA, Raval MJ, Martin GR, Kolb J, Afrouzian M, Buie WD, Sigalet DL (2005) The effects of systemic hypoxia on colon anastomotic healing: an animal model. Dis Colon Rectum 48(7):1460–1470. https://doi.org/10.1007/s10350-005-0047-3
Hirano Y, Omura K, Tatsuzawa Y, Shimizu J, Kawaura Y, Watanabe G (2006) Tissue oxygen saturation during colorectal surgery measured by near-infrared spectroscopy: pilot study to predict anastomotic complications. World J Surg 30(3):457–461. https://doi.org/10.1007/s00268-005-0271-y
Karliczek A, Benaron DA, Baas PC, Zeebregts CJ, Wiggers T, van Dam GM (2010) Intraoperative assessment of microperfusion with visible light spectroscopy for prediction of anastomotic leakage in colorectal anastomoses. Colorectal Dis Off J Assoc Coloproctol G B Irel 12(10):1018–1025. https://doi.org/10.1111/j.1463-1318.2009.01944.x
Millan M, Garcia-Granero E, Flor B, Garcia-Botello S, Lledo S (2006) Early prediction of anastomotic leak in colorectal cancer surgery by intramucosal pH. Dis Colon Rectum 49(5):595–601. https://doi.org/10.1007/s10350-006-0504-7
Urbanavicius L, Pattyn P, de Putte DV, Venskutonis D (2011) How to assess intestinal viability during surgery: a review of techniques. World J Gastrointest Surg 3(5):59–69. https://doi.org/10.4240/wjgs.v3.i5.59
Jafari MD, Lee KH, Halabi WJ, Mills SD, Carmichael JC, Stamos MJ, Pigazzi A (2013) The use of indocyanine green fluorescence to assess anastomotic perfusion during robotic assisted laparoscopic rectal surgery. Surg Endosc 27(8):3003–3008. https://doi.org/10.1007/s00464-013-2832-8
Jafari MD, Wexner SD, Martz JE, McLemore EC, Margolin DA, Sherwinter DA, Lee SW, Senagore AJ, Phelan MJ, Stamos MJ (2015) Perfusion assessment in laparoscopic left-sided/anterior resection (PILLAR II): a multi-institutional study. J Am Coll Surg 220(1):82–92 e81. https://doi.org/10.1016/j.jamcollsurg.2014.09.015
Milstein DM, Ince C, Gisbertz SS, Boateng KB, Geerts BF, Hollmann MW, van Berge Henegouwen MI, Veelo DP (2016) Laser speckle contrast imaging identifies ischemic areas on gastric tube reconstructions following esophagectomy. Medicine 95(25):e3875. https://doi.org/10.1097/MD.0000000000003875
De Backer D, Hollenberg S, Boerma C, Goedhart P, Buchele G, Ospina-Tascon G, Dobbe I, Ince C (2007) How to evaluate the microcirculation: report of a round table conference. Crit Care 11(5):R101. https://doi.org/10.1186/cc6118
de Bruin AF, Kornmann VN, van der Sloot K, van Vugt JL, Gosselink MP, Smits A, Van Ramshorst B, Boerma EC, Noordzij PG, Boerma D, van Iterson M (2016) Sidestream dark field imaging of the serosal microcirculation during gastrointestinal surgery. Colorectal Dis Off J Assoc Coloproctol G B Irel. https://doi.org/10.1111/codi.13250
de Bruin AF, Tavy A, van der Sloot K, Smits A, Van Ramshorst B, Boerma CE, Kars P, Noordzij PG, Boerma D, van Iterson M (2016) Use of an image acquisition stabilizer improves sidestream dark field imaging of the serosa during open gastrointestinal surgery. J Vasc Res 53(3–4):121–127. https://doi.org/10.1159/000448735
Balestra GM, Bezemer R, Boerma EC, Yong ZY, Sjauw KD, Engstrom AE, Koopmans M, Ince C (2010) Improvement of sidestream dark field imaging with an image acquisition stabilizer. BMC Med Imaging 10:15. https://doi.org/10.1186/1471-2342-10-15
Lindert J, Werner J, Redlin M, Kuppe H, Habazettl H, Pries AR (2002) OPS imaging of human microcirculation: a short technical report. J Vasc Res 39(4):368–372. https://doi.org/10.1159/000065549
Goedhart PT, Khalilzada M, Bezemer R, Merza J, Ince C (2007) Sidestream dark field (SDF) imaging: a novel stroboscopic LED ring-based imaging modality for clinical assessment of the microcirculation. Opt Express 15(23):15101–15114. https://doi.org/10.1364/OE.15.015101
Aykut G, Ince y, Ince C (2014) A new generation computer-controlled imaging sensor-based hand-held microscope for quantifying bedside microcirculatory alterations. In: Vincent L (ed) Annual update in intensive care and emergency medicine 2014. Springer International Publishing, Geneva. https://doi.org/10.1007/978-3-319-03746-2_28
Trzeciak S, Dellinger RP, Parrillo JE, Guglielmi M, Bajaj J, Abate NL, Arnold RC, Colilla S, Zanotti S, Hollenberg SM (2007) Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med 49(1):88–98. https://doi.org/10.1016/j.annemergmed.2006.08.021 (98 e81–82)
Dobbe JG, Streekstra GJ, Atasever B, van Zijderveld R, Ince C (2008) Measurement of functional microcirculatory geometry and velocity distributions using automated image analysis. Med Biol Eng Comput 46(7):659–670. https://doi.org/10.1007/s11517-008-0349-4
Karliczek A, Harlaar NJ, Zeebregts CJ, Wiggers T, Baas PC, van Dam GM (2009) Surgeons lack predictive accuracy for anastomotic leakage in gastrointestinal surgery. Int J Colorectal Dis 24(5):569–576. https://doi.org/10.1007/s00384-009-0658-6
Kashiwagi H (1993) The lower limit of tissue blood flow for safe colonic anastomosis: an experimental study using laser Doppler velocimetry. Surg Today 23(5):430–438
Schouten SB, De Bruin AF, Gosselink MP, Nigg AL, van Iterson M, Biermann K, Kliffen M, van der Harst E (2014) Is microvessel density correlated with anastomotic leakage after low anterior resection? Hepato-gastroenterology 61(129):90–93
Vignali A, Gianotti L, Braga M, Radaelli G, Malvezzi L, Di Carlo V (2000) Altered microperfusion at the rectal stump is predictive for rectal anastomotic leak. Dis Colon Rectum 43(1):76–82
Kingham TP, Pachter HL (2009) Colonic anastomotic leak: risk factors, diagnosis, and treatment. J Am Coll Surg 208(2):269–278. https://doi.org/10.1016/j.jamcollsurg.2008.10.015
McDermott FD, Heeney A, Kelly ME, Steele RJ, Carlson GL, Winter DC (2015) Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg 102(5):462–479. https://doi.org/10.1002/bjs.9697
Edul VS, Enrico C, Laviolle B, Vazquez AR, Ince C, Dubin A (2012) Quantitative assessment of the microcirculation in healthy volunteers and in patients with septic shock. Crit Care Med 40(5):1443–1448. https://doi.org/10.1097/CCM.0b013e31823dae59
Ince C (2005) The microcirculation is the motor of sepsis. Crit Care 9(Suppl 4):S13–S19. https://doi.org/10.1186/cc3753
Guraieb-Trueba M, Frering T, Atallah S (2016) Combined endoscopic and laparoscopic real-time intra-operative evaluation of bowel perfusion using fluorescence angiography. Tech Coloproctol 20:883–884-. https://doi.org/10.1007/s10151-016-1547-y
Kudszus S, Roesel C, Schachtrupp A, Hoer JJ (2010) Intraoperative laser fluorescence angiography in colorectal surgery: a noninvasive analysis to reduce the rate of anastomotic leakage. Langenbeck’s archives of surgery. Deutsche Gesellschaft fur Chirurgie 395(8):1025–1030. https://doi.org/10.1007/s00423-010-0699-x
Mizrahi I, Abu-Gazala M, Rickles AS, Fernandez LM, Petrucci A, Wolf J, Sands DR, Wexner SD (2018) Indocyanine green fluorescence angiography during low anterior resection for low rectal cancer: results of a comparative cohort study. Tech Coloproctol 22:535–540. https://doi.org/10.1007/s10151-018-1832-z (Epub 2018 Aug 10)
Ris F, Hompes R, Cunningham C, Lindsey I, Guy R, Jones O, George B, Cahill RA, Mortensen NJ (2014) Near-infrared (NIR) perfusion angiography in minimally invasive colorectal surgery. Surg Endosc 28(7):2221–2226. https://doi.org/10.1007/s00464-014-3432-y
Siegemund M, van Bommel J, Stegenga ME, Studer W, van Iterson M, Annaheim S, Mebazaa A, Ince C (2010) Aortic cross-clamping and reperfusion in pigs reduces microvascular oxygenation by altered systemic and regional blood flow distribution. Anesth Analg 111(2):345–353. https://doi.org/10.1213/ANE.0b013e3181e4255f
Nakatsuka M (2002) Assessment of gut mucosal perfusion and colonic tissue blood flow during abdominal aortic surgery with gastric tonometry and laser Doppler flowmetry. Vasc Endovasc Surg 36(3):193–198
Singh DB, Stansby G, Bain I, Harrison DK (2009) Intraoperative measurement of colonic oxygenation during bowel resection. Adv Exp Med Biol 645:261–266. https://doi.org/10.1007/978-0-387-85998-9_39
Boerma EC, van der Voort PH, Spronk PE, Ince C (2007) Relationship between sublingual and intestinal microcirculatory perfusion in patients with abdominal sepsis. Crit Care Med 35(4):1055–1060. https://doi.org/10.1097/01.CCM.0000259527.89927.F9
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was performed at a tertiary teaching hospital (St Antonius Hospital, Nieuwegein, the Netherlands) after approval from the united medical ethics committees (NL48332.100.14/R14.005).
Informed consent
All patients gave their written informed consent as per the applicable laws.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (AVI 12458 KB)
Rights and permissions
About this article
Cite this article
de Bruin, A.F.J., Tavy, A.L.M., van der Sloot, K. et al. Can sidestream dark field (SDF) imaging identify subtle microvascular changes of the bowel during colorectal surgery?. Tech Coloproctol 22, 793–800 (2018). https://doi.org/10.1007/s10151-018-1872-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10151-018-1872-4