Abstract
Background
The outcomes of relapsed or refractory acute myeloid leukemia (AML) remain poor. Although the concomitant use of granulocyte colony-stimulating factor (G-CSF) and anti-chemotherapeutic agents has been investigated to improve the antileukemic effect on AML, its usefulness remains controversial. This study aimed to investigate the effects of G-CSF priming as a remission induction therapy or salvage chemotherapy.
Methods
We performed a thorough literature search for studies related to the priming effect of G-CSF using PubMed, Ichushi-Web, and the Cochrane Library. A qualitative analysis of the pooled data was performed, and risk ratios (RRs) with confidence intervals (CIs) were calculated and summarized.
Results
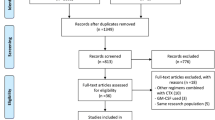
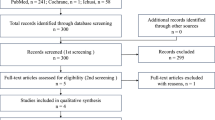
Two reviewers independently extracted and accessed the 278 records identified during the initial screening, and 62 full-text articles were assessed for eligibility in second screening. Eleven studies were included in the qualitative analysis and 10 in the meta-analysis. A systematic review revealed that priming with G-CSF did not correlate with an improvement in response rate and overall survival (OS). The result of the meta-analysis revealed the tendency for lower relapse rate in the G-CSF priming groups without inter-study heterogeneity [RR, 0.91 (95% CI 0.82–1.01), p = 0.08; I2 = 4%, p = 0.35]. In specific populations, including patients with intermediate cytogenetic risk and those receiving high-dose cytarabine, the G-CSF priming regimen prolonged OS.
Conclusions
G-CSF priming in combination with intensive remission induction treatment is not universally effective in patients with AML. Further studies are required to identify the patient cohort for which G-CSF priming is recommended.




Similar content being viewed by others
Data availability
Data associated with this systematic review may be accessed from the corresponding author upon reasonable request.
References
Döhner H, Weisdorf DJ, Bloomfield CD (2015) Acute myeloid leukemia. N Engl J Med 373(12):1136–1152. https://doi.org/10.1056/NEJMra1406184
Ishikawa F, Yoshida S, Saito Y et al (2007) Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol 25(11):1315–1321. https://doi.org/10.1038/nbt1350
Lapidot T, Sirard C, Vormoor J et al (1994) A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367(6464):645–648. https://doi.org/10.1038/367645a0
Bonnet D, Dick JE (1997) Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 3(7):730–737. https://doi.org/10.1038/nm0797-730
Akiyama N, Okamura T, Yoshida M et al (2022) Difference of compliance rates for the recommendations in Japanese Guideline on Febrile Neutropenia according to respondents’ attributes: the second report on a questionnaire survey among hematology-oncology physicians and surgeons. Support Care Cancer 30(5):4327–4336. https://doi.org/10.1007/s00520-022-06834-9
Pahnke S, Egeland T, Halter J et al (2019) Current use of biosimilar G-CSF for haematopoietic stem cell mobilisation. Bone Marrow Transplant 54(6):858–866. https://doi.org/10.1038/s41409-018-0350-y
Arora S, Majhail NS, Liu H (2019) Hematopoietic progenitor cell mobilization for autologous stem cell transplantation in multiple myeloma in contemporary era. Clin Lymphoma Myeloma Leuk 19(4):200–205. https://doi.org/10.1016/j.clml.2018.12.010
Hartmann T, Hübel K, Monsef I et al (2015) Additional plerixafor to granulocyte colony-stimulating factors for haematopoietic stem cell mobilisation for autologous transplantation in people with malignant lymphoma or multiple myeloma. Cochrane Database Syst Rev 2015(10):Cd010615. https://doi.org/10.1002/14651858.CD010615.pub2
Terpstra W, Löwenberg B (1997) Application of myeloid growth factors in the treatment of acute myeloid leukemia. Leukemia 11(3):315–327. https://doi.org/10.1038/sj.leu.2400561
Saito Y, Uchida N, Tanaka S et al (2010) Induction of cell cycle entry eliminates human leukemia stem cells in a mouse model of AML. Nat Biotechnol 28(3):275–280. https://doi.org/10.1038/nbt.1607
Löwenberg B, van Putten W, Theobald M et al (2003) Effect of priming with granulocyte colony-stimulating factor on the outcome of chemotherapy for acute myeloid leukemia. N Engl J Med 349(8):743–752. https://doi.org/10.1056/NEJMoa025406
Amadori S, Suciu S, Jehn U et al (2005) Use of glycosylated recombinant human G-CSF (lenograstim) during and/or after induction chemotherapy in patients 61 years of age and older with acute myeloid leukemia: final results of AML-13, a randomized phase-3 study. Blood 106(1):27–34. https://doi.org/10.1182/blood-2004-09-3728
Milligan DW, Wheatley K, Littlewood T et al (2006) Fludarabine and cytosine are less effective than standard ADE chemotherapy in high-risk acute myeloid leukemia, and addition of G-CSF and ATRA are not beneficial: results of the MRC AML-HR randomized trial. Blood 107(12):4614–4622. https://doi.org/10.1182/blood-2005-10-4202
Krug U, Berdel WE, Gale RP et al (2016) Increasing intensity of therapies assigned at diagnosis does not improve survival of adults with acute myeloid leukemia. Leukemia 30(6):1230–1236. https://doi.org/10.1038/leu.2016.25
Ohno R, Naoe T, Kanamaru A et al (1994) A double-blind controlled study of granulocyte colony-stimulating factor started two days before induction chemotherapy in refractory acute myeloid leukemia. Kohseisho Leukemia Study Group Blood 83(8):2086–2092
Pabst T, Vellenga E, van Putten W et al (2012) Favorable effect of priming with granulocyte colony-stimulating factor in remission induction of acute myeloid leukemia restricted to dose escalation of cytarabine. Blood 119(23):5367–5373. https://doi.org/10.1182/blood-2011-11-389841
Wilson A, Laurenti E, Oser G et al (2008) Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135(6):1118–1129. https://doi.org/10.1016/j.cell.2008.10.048
National Comprehensive Cancer Network Acute Myeloid Leukemia (Version 4.2023). https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf. Accessed 7 Aug 2023
Morizane T, Yoshida M, Kojimahara N et al (2014) Minds Handbook for Clinical Practice Guideline Development 2014. Japan Council for Quality Health Care, Tokyo. https://minds.jcqhc.or.jp/s/developer_manual(in Japanese)
Kojimahara N, Nakayama T, Morizane T et al (2017) Minds Manual for Guideline Development 2017. Japan Council for Quality Health Care, Tokyo
Borthakur G, Kantarjian H, Wang X et al (2008) Treatment of core-binding-factor in acute myelogenous leukemia with fludarabine, cytarabine, and granulocyte colony-stimulating factor results in improved event-free survival. Cancer 113(11):3181–3185. https://doi.org/10.1002/cncr.23927
Estey E, Thall P, Andreeff M et al (1994) Use of granulocyte colony-stimulating factor before, during, and after fludarabine plus cytarabine induction therapy of newly diagnosed acute myelogenous leukemia or myelodysplastic syndromes: comparison with fludarabine plus cytarabine without granulocyte colony-stimulating factor. J Clin Oncol 12(4):671–678. https://doi.org/10.1200/jco.1994.12.4.671
Halpern AB, Othus M, Huebner EM et al (2018) Phase 1/2 trial of GCLAM with dose-escalated mitoxantrone for newly diagnosed AML or other high-grade myeloid neoplasms. Leukemia 32(11):2352–2362. https://doi.org/10.1038/s41375-018-0135-8
Martin MG, Augustin KM, Uy GL et al (2009) Salvage therapy for acute myeloid leukemia with fludarabine, cytarabine, and idarubicin with or without gemtuzumab ozogamicin and with concurrent or sequential G-CSF. Am J Hematol 84(11):733–737. https://doi.org/10.1002/ajh.21545
Vulaj V, Perissinotti AJ, Uebel JR et al (2018) The FOSSIL Study: FLAG or standard 7+3 induction therapy in secondary acute myeloid leukemia. Leuk Res 70:91–96. https://doi.org/10.1016/j.leukres.2018.05.011
te Boekhorst PA, Löwenberg B, Vlastuin M et al (1993) Enhanced chemosensitivity of clonogenic blasts from patients with acute myeloid leukemia by G-CSF, IL-3 or GM-CSF stimulation. Leukemia 7(8):1191–1198
Konuma T, Kato S, Isobe M et al (2019) Reduced-toxicity myeloablative conditioning consisting of fludarabine/busulfan/low-dose total body irradiation/granulocyte colony-stimulating factor-combined cytarabine in single cord blood transplantation for elderly patients with nonremission myeloid malignancies. Biol Blood Marrow Transplant 25(4):764–770. https://doi.org/10.1016/j.bbmt.2018.12.004
Konuma T, Takahashi S, Uchida N et al (2015) Effect of Granulocyte Colony-Stimulating Factor-Combined Conditioning in Cord Blood Transplantation for Myelodysplastic Syndrome and Secondary Acute Myeloid Leukemia: A Retrospective Study in Japan. Biol Blood Marrow Transplant 21(9):1632–1640. https://doi.org/10.1016/j.bbmt.2015.05.009
Mori T, Aisa Y, Watanabe R et al (2008) Long-term follow-up of allogeneic hematopoietic stem cell transplantation for de novo acute myelogenous leukemia with a conditioning regimen of total body irradiation and granulocyte colony-stimulating factor-combined high-dose cytarabine. Biol Blood Marrow Transplant 14(6):651–657. https://doi.org/10.1016/j.bbmt.2008.03.006
Takahashi S, Iseki T, Ooi J et al (2004) Single-institute comparative analysis of unrelated bone marrow transplantation and cord blood transplantation for adult patients with hematologic malignancies. Blood 104(12):3813–3820. https://doi.org/10.1182/blood-2004-03-1001
Terakura S, Konuma T, Tanaka M et al (2020) Randomised controlled trial of conditioning regimen for cord blood transplantation for adult myeloid malignancies comparing high-dose cytarabine/cyclophosphamide/total body irradiation with versus without G-CSF priming: G-CONCORD study protocol. BMJ Open 10(12):e040467. https://doi.org/10.1136/bmjopen-2020-040467
Yamada K, Furusawa S, Saito K et al (1995) Concurrent use of granulocyte colony-stimulating factor with low-dose cytosine arabinoside and aclarubicin for previously treated acute myelogenous leukemia: a pilot study. Leukemia 9(1):10–14
Huang J, Hong M, Zhu Y (2018) Decitabine in combination with G-CSF, low-dose cytarabine and aclarubicin is as effective as standard dose chemotherapy in the induction treatment for patients aged from 55 to 69 years old with newly diagnosed acute myeloid leukemia. Leuk Lymphoma 59(11):2570–2579. https://doi.org/10.1080/10428194.2018.1443328
Chen X, Zhao Y, Li Q et al (2023) Single-center retrospective clinical evaluation of venetoclax combined with HMAs and half-dose CAG for unfit or refractory/relapsed AML. Onco Targets Ther 16:409–419. https://doi.org/10.2147/ott.S405611
Hatsumi N, Miyawaki S, Yamauchi T et al (2019) Phase II study of FLAGM (fludarabine + high-dose cytarabine + granulocyte colony-stimulating factor + mitoxantrone) for relapsed or refractory acute myeloid leukemia. Int J Hematol 109(4):418–425. https://doi.org/10.1007/s12185-019-02606-0
Acknowledgements
The authors are grateful to Ms. Natsuki Narita for her contribution to the initial literature search. The authors thank Ms. Natsuki Fukuda for her valuable comments and suggestions. We would like to thank Editage (www.editage.jp) for English language editing.
Funding
This study was partially supported by the Japan Society for the Promotion of Science, KAKENHI (grant number: JP22K15615).
Author information
Authors and Affiliations
Contributions
All the authors contributed to the conception and design of this present study. Y.N. wrote the draft of the manuscript, and all authors reviewed and commented on the manuscript. All the authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
T. Maeda reports scholarship donation from Chugai Pharmaceutical. SN reports honoraria from Kyowa Kirin. YO reports honoraria from Daiichi Sankyo, Pfizer, Chugai Pharmaceutical, Eli Lilly and Company, and Kyowa Kirin. KT reports honoraria from Ono Pharmaceutical, Chugai Pharmaceutical, Taiho Pharmaceutical, and Novartis Pharma. EI reports honoraria from Eli Lilly and Company, research funding from MSD, Ono Pharmaceutical, Jannsen Pharma, and Takeda Pharmaceutical. YM reports honoraria from Ono Pharmaceutical, MSD, Takeda Pharmaceutical, Eisai, Bristol Myers Squibb, research funding from MSD, and Ono Pharmaceutical. DM reports honoraria from Ono Pharmaceutical, Janssen Pharma, Nippon Shinyaku, Eisai, Mundipharma, Kyowa Kirin, Chugai Pharmaceutical, Zenyaku, MSD, SymBio Pharmaceuticals, Sanofi, AbbVie, Takeda Pharmaceutical, AstraZeneca, Bristol Myers Squibb, Genmab, research funding from Amgen Astellas Biopharma, Novartis Pharma, Kyowa Kirin, Ono Pharmaceutical, Chugai Pharmaceutical, Janssen Pharma, Takeda Pharmaceutical, Otsuka Pharmaceutical, Sanofi, Astellas, Bristol Myers Squibb, AbbVie, Eisai, MSD, Taiho Pharmaceutical, AstraZeneca, Eli Lilly and Company, and Genmab. TY reports honoraria from Kyowa Kirin, Pfizer, Chugai Pharmaceutical, Eli Lilly and Company, MSD, AstraZeneca, and Eisai. T. Motohashi reports honoraria from AstraZeneca, Chugai Pharmaceutical, and Myriad Genetics. EB reports honoraria from Chugai Pharmaceutical, Daiichi Sankyo, research funding from Taiho Pharmaceutical, and Chugai Pharmaceutical. T. Kubo reports honoraria from Chugai Pharmaceutical. T. Kimura reports honoraria from Sanofi. AS reports honoraria and research funding from Chugai Pharmaceutical, and Taiho Pharmaceutical. TT reports honoraria from Daiichi Sankyo, Chugai Pharmaceutical, and Eli Lilly and Company. SY reports research funding from Otsuka Pharmaceutical. The other authors declare that there are no conflicts of interest associated with this manuscript.
Ethical approval
Not applicable.
Informed consent
Formal consent was not required for this type of study.
Consent to participate
Not applicable.
Consent for publication
All authors consented to the publication of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Najima, Y., Maeda, T., Kamiyama, Y. et al. Effectiveness and safety of granulocyte colony-stimulating factor priming regimen for acute myeloid leukemia: A systematic review and meta-analysis of the Clinical Practice Guideline for the use of G-CSF 2022 from the Japan Society of Clinical Oncology. Int J Clin Oncol (2024). https://doi.org/10.1007/s10147-023-02461-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10147-023-02461-4