Abstract
Background
Definitive chemoradiotherapy (CRT) with 5-fluorouracil plus mitomycin-C is a standard treatment for stage II/III squamous cell carcinoma of the anal canal (SCCA). We performed this dose-finding and single-arm confirmatory trial of CRT with S-1 plus mitomycin-C to determine the recommended dose (RD) of S-1 and evaluate its efficacy and safety for locally advanced SCCA.
Methods
Patients with clinical stage II/III SCCA (UICC 6th) received CRT comprising mitomycin-C (10 mg/m2 on days 1 and 29) and S-1 (60 mg/m2/day at level 0 and 80 mg/m2/day at level 1 on days 1–14 and 29–42) with concurrent radiotherapy (59.4 Gy). Dose-finding used a 3 + 3 cohort design. The primary endpoint of the confirmatory trial was 3-year event-free survival. The sample size was 65, with one-sided alpha of 5%, power of 80%, and expected and threshold values of 75% and 60%, respectively.
Results
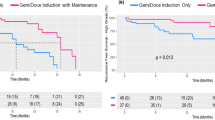
Sixty-nine patients (dose-finding, n = 10; confirmatory, n = 59) were enrolled. The RD of S-1 was determined as 80 mg/m2/day. Three-year event-free survival in 63 eligible patients who received the RD was 65.0% (90% confidence interval 54.1–73.9). Three-year overall, progression-free, and colostomy-free survival rates were 87.3%, 85.7%, and 76.2%, respectively; the complete response rate was 81% on central review. Common grade 3/4 acute toxicities were leukopenia (63.1%), neutropenia (40.0%), diarrhea (20.0%), radiation dermatitis (15.4%), and febrile neutropenia (3.1%). No treatment-related deaths occurred.
Conclusions
Although the primary endpoint was not met, S-1/mitomycin-C chemoradiotherapy had an acceptable toxicity profile and favorable 3-year survival and could be a treatment option for locally advanced SCCA.
Clinical trial information
jRCTs031180002.


Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69:7–34
Hotta T (2018) Cancer statistics in Japan 2018. Foundation for Promotion of Cancer Research, Tokyo
UKCCCR Anal Cancer Working Party (1996) Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil and mitomycin. Lancet 348:1049–1054
Northover J, Glynne-Jones R, Sebag-Montefi Ore D et al (2010) Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR anal cancer trial (ACT I). Br J Cancer 102:1123–1128
Bartelink H, Roelofsen F, Eschwege F et al (1997) Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the european organization for research and treatment of cancer radiotherapy and gastrointestinal cooperative groups. J Clin Oncol 15:2040–2049
Flam M, John M, Pajak TF et al (1996) Role of mitomycin in combination with fluorouracil and radiotherapy and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol 14:2527–2539
Ajani JA, Winter KA, Gunderson LL et al (2008) Fluorouracil, mitomycin and radiotherapy vs fluorouracil, cisplatin and radiotherapy for carcinoma of the anal canal: a randomised controlled trial. JAMA 199:1914–1921
Gunderson LL, Winter KA, Ajani JA et al (2012) Long-term update of U.S. GI Intergroup RTOG 98–11 phase III trial for anal carcinoma: survival, relapse and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluoruracil/cisplatin. J Clin Oncol 30:4344–4351
James RD, Glynne-Jones R, Meadows HM et al (2013) Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2 × 2 factorial trial. Lancet Oncol 14:516–524
Takashima A, Shimada Y, Hamaguchi T et al (2009) Current therapeutic strategies for anal squamous cell carcinoma in Japan. Int J Clin Oncol 14:416–420
Boku N, Yamamoto S, Fukuda H et al (2009) Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol 10:1063–1069
Zeng L, Ou G, Itasaka S et al (2008) TS-1 enhances the effect of radiotherapy by suppressing radiation-induced hypoxia-inducible factor-1 activation and inducing endothelial cell apoptosis. Cancer Sci 99:2327–2335
Takashima A, Shimada Y, Hamaguchi T et al (2011) A Phase I/II trial of chemoradiotherapy concurrent with S-1 plus mitomycin C in patients with clinical stage II/III squamous cell carcinoma of anal canal (JCOG0903: SMART-AC). Jpn J Clin Oncol 41:713–717
Tahara M, Fuse N, Mizusawa J et al (2015) Phase I/II trial of chemoradiotherapy with concurrent S-1 and cisplatin for clinical stage II/III esophageal carcinoma (JCOG 0604). Cancer Sci 106:1414–1420
Park SH, Kim YS, Hong J et al (2008) Mitomycin C plus S-1 as second-line therapy in patients with advanced gastric cancer: a noncomparative phase II study. Anticancer Drugs 19:303–307
Glynne-Jones R, Sebag-Montefiore D, Meadows HM et al (2017) Best time to assess complete clinical response after chemoradiotherapy in squamous cell carcinoma of the anus (ACT II): a post-hoc analysis of randomised controlled phase 3 trial. Lancet Oncol 18:347–356
Glynne-Jones R, Meadows H, Wan S et al (2008) EXTRA–a multicenter phase II study of chemoradiation using a 5 day per week oral regimen of capecitabine and intravenous mitomycin C in anal cancer. Int J Radiat Oncol Biol Phys 72:119–126
National Comprehensive Cancer Network (NCCN) Clinical Practice guidelines in oncology. Anal carcinoma 2021; Version 1. http://www.nccn.org/professionals/physician_gls/PDF/anal.pdf. Accessed 10 May 2021
Morris VK, Salem ME, Nimeiri H et al (2017) Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): a multicentre, single-arm, phase 2 study. Lancet Oncol 18:446–453
Ott PA, Piha-Paul SA, Munster P et al (2017) Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with recurrent carcinoma of the anal canal. Ann Oncol 28:1036–1041
Antonia SJ, Villegas A, Daniel D et al (2017) Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 377:1919–1929
Antonia SJ, Villegas A, Daniel D et al (2018) Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 379:2342–2350
Kachnic L, Winter K, Myerson R et al (2013) RTOG 0529: a phase II evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys 86:27–33
Acknowledgements
We thank all the patients and their families, and all staff in participating institutions and at the JCOG Data Center and Operations Office. JCOG Data Center and Operations Office: Ayaka Nakano and Michiko Murai (Data Management Section), and Junko Eba (Operations Office). We also thank Yoshihiro Moriya (first study group chair of Colorectal Cancer study Group at the start of this trial) for his encouragement and for supporting this trial. We also thank Editage and ThinkSCIENCE for English language editing. Part of the data was presented at the 2019 Gastrointestinal Cancers Symposium, held in San Francisco, CA, USA in 2019.
Funding
This study was supported in part by the National Cancer Center Research and Development Funds (26-A-4, 29-A-3, 2020-J-3, 2023-J-03), and Grants-in-Aid for Clinical Cancer Research (H23Gann-012) from the Ministry of Health, Labour and Welfare of Japan.
Author information
Authors and Affiliations
Consortia
Contributions
Y, TH, AT, JM, YS, HK, and YK conceived the study design. YI, TH, AT, YS, MS, NM, TK, KK, MO, KK, FT, MK, KM, FF, MW, and YK contributed to data collection. YI, TH, AT, JM, YS, HK, HF, and YK analyzed the data and interpreted the results. All authors wrote manuscript and approved the final version. All authors had accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Conflict of interest
Tetsuya Hamaguchi has received honoraria and research funding from Taiho Pharmaceutical. All other authors have no disclosures to declare.
Ethical approval
The study protocol was reviewed and approved by the Protocol Review Committee of JCOG and the institutional review board at each institution.
Consent to participate
All patients provided written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Ito, Y., Hamaguchi, T., Takashima, A. et al. Definitive S-1/mitomycin-C chemoradiotherapy for stage II/III anal canal squamous cell carcinoma: a phase I/II dose-finding and single-arm confirmatory study (JCOG0903). Int J Clin Oncol 28, 1063–1072 (2023). https://doi.org/10.1007/s10147-023-02361-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-023-02361-7