Abstract
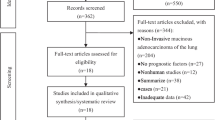
The prognostic value of myosteatosis has been widely investigated in lung cancer, yet conclusions remain controversial. The purpose of this meta-analysis was to illuminate this issue. Medline, Embase, Cochrane Library and Web of Science Core Collection online databases were systematically searched from inception to 24 September 2021. Newcastle–Ottawa Scale tool was applied to evaluate the quality of included studies. Pooled hazard ratios (HRs) with 95% confidence intervals (CIs) for overall survival (OS) and progression-free survival (PFS) were used to examine prognostic value of myosteatosis. Subgroup analysis and sensitivity analysis were conducted to assess heterogeneity and stability of results. A total of 484 articles were screened from which 9 eligible studies involving 1667 patients were enrolled in this meta-analysis. Lung cancer patients with myosteatosis had significantly worse OS than patients without myosteatosis (HR 1.10, 95% CI 1.05–1.16, P < 0.001), both in six multivariate analysis (HR 1.46, 95% CI 1.16–1.85, P = 0.001) and in three univariate analysis (HR 1.08, 95% CI 1.03–1.14, P = 0.003). Pooled data from five studies using multivariate survival analysis also showed that patients with myosteatosis had a statistically significant unfavorable PFS (HR = 1.27, 95% CI 1.00–1.62, P = 0.049). Sensitivity analysis showed the result for OS was stable. But for PFS, the result was not robust. Myosteatosis might serve as an independent indicator of unfavorable survival outcomes for OS and PFS in lung cancer patients. Further studies are needed to confirm our results.





Similar content being viewed by others
References
Thai AA, Solomon BJ, Sequist LV et al (2021) Lung cancer. Lancet 398(10299):535–554. https://doi.org/10.1016/S0140-6736(21)00312-3
Woodard GA, Jones KD, Jablons DM (2016) Lung cancer staging and prognosis. Cancer Treat Res 170:47–75. https://doi.org/10.1007/978-3-319-40389-2_3
Wang A, Wang HY, Liu Y et al (2015) The prognostic value of PD-L1 expression for non-small cell lung cancer patients: a meta-analysis. Eur J Surg Oncol 41(4):450–456. https://doi.org/10.1016/j.ejso.2015.01.020
Simmons CP, Koinis F, Fallon MT et al (2015) Prognosis in advanced lung cancer–a prospective study examining key clinicopathological factors. Lung Cancer 88(3):304–309. https://doi.org/10.1016/j.lungcan.2015.03.020
Martin L, Birdsell L, Macdonald N et al (2013) Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 31(12):1539–1547. https://doi.org/10.1200/jco.2012.45.2722
Grønberg BH, Sjøblom B, Wentzel-Larsen T et al (2019) A comparison of CT based measures of skeletal muscle mass and density from the Th4 and L3 levels in patients with advanced non-small-cell lung cancer. Eur J Clin Nutr 73(7):1069–1076. https://doi.org/10.1038/s41430-018-0325-5
Amini B, Boyle SP, Boutin RD et al (2019) Approaches to assessment of muscle mass and myosteatosis on computed tomography: a systematic review. J Gerontol A Biol Sci Med Sci 74(10):1671–1678. https://doi.org/10.1093/gerona/glz034
Goodpaster BH, Carlson CL, Visser M et al (2001) Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol 90(6):2157–2165. https://doi.org/10.1152/jappl.2001.90.6.2157
Correa-de-Araujo R, Addison O, Miljkovic I et al (2020) Myosteatosis in the context of skeletal muscle function deficit: an interdisciplinary workshop at the national institute on aging. Front Physiol 11:963. https://doi.org/10.3389/fphys.2020.00963
Goodpaster BH, Kelley DE, Thaete FL et al (2000) Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol 89(1):104–110. https://doi.org/10.1152/jappl.2000.89.1.104
Okugawa Y, Orcid Id, Toiyama Y et al (2018) Clinical impact of muscle quantity and quality in colorectal cancer patients: a propensity score matching analysis. JPEN J Parenter Enteral Nutr 42(8):1322–1333. https://doi.org/10.1002/jpen.1171
Pozzuto L, Silveira MN, Mendes MCS et al (2021) Myosteatosis differentially affects the prognosis of non-metastatic colon and rectal cancer patients: an exploratory study. Front Oncol 11:762444. https://doi.org/10.3389/fonc.2021.762444
Lee CM, Kang J (2020) Prognostic impact of myosteatosis in patients with colorectal cancer: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 11(5):1270–1282. https://doi.org/10.1002/jcsm.12575
Aleixo GFP, Orcid Id, Williams GR et al (2019) Muscle composition and outcomes in patients with breast cancer: meta-analysis and systematic review. Breast Cancer Res Treat 177(3):569–579. https://doi.org/10.1007/s10549-019-05352-3
McSharry V, Mullee A, McCann L et al (2020) The impact of sarcopenia and low muscle attenuation on overall survival in epithelial ovarian cancer: a systematic review and meta-analysis. Ann Surg Oncol 27(9):3553–3564. https://doi.org/10.1245/s10434-020-08382-0
Cortellini A, Orcid Id, Palumbo P et al (2018) Single-institution study of correlations between skeletal muscle mass, its density, and clinical outcomes in non-small cell lung cancer patients treated with first-line chemotherapy. Thorac Cancer 9(12):1623–1630. https://doi.org/10.1111/1759-7714.12870
Bowden JCS, Williams LJ, Simms A et al (2017) Prediction of 90 day and overall survival after chemoradiotherapy for lung cancer: role of performance status and body composition. Clin Oncol 29(9):576–584. https://doi.org/10.1016/j.clon.2017.06.005
Nattenmüller J, Wochner R, Muley T et al (2017) Prognostic impact of CT-quantified muscle and fat distribution before and after first-line-chemotherapy in lung cancer patients. PLoS ONE 12(1):e0169136. https://doi.org/10.1371/journal.pone.0169136
Nishioka N, Naito T, Orcid Id et al (2021) Unfavorable impact of decreased muscle quality on the efficacy of immunotherapy for advanced non-small cell lung cancer. Cancer Med 10(1):247–256. https://doi.org/10.1002/cam4.3631
Kiss N, Orcid Id, Beraldo J et al (2019) Early skeletal muscle loss in non-small cell lung cancer patients receiving chemoradiation and relationship to survival. Support Care Cancer 27(7):2657–2664. https://doi.org/10.1007/s00520-018-4563-9
Minami S, Ihara S, Nishimatsu K et al (2019) Low body mass index is an independent prognostic factor in patients with non-small cell lung cancer treated with epidermal growth factor receptor tyrosine kinase inhibitor. World J Oncol 10(6):187–198. https://doi.org/10.14740/wjon1244
Minami S, Ihara S, Komuta K (2020) Sarcopenia and visceral adiposity are not independent prognostic markers for extensive disease of small-cell lung cancer: a single-centered retrospective cohort study. World J Oncol 11(4):139–149. https://doi.org/10.14740/wjon1289
Minami S, Ihara S, Tanaka T et al (2020) Sarcopenia and visceral adiposity did not affect efficacy of immune-checkpoint inhibitor monotherapy for pretreated patients with advanced non-small cell lung cancer. World J Oncol 11(1):9–22. https://doi.org/10.14740/wjon1225
Abbass T, Dolan RD, MacLeod N et al (2020) Comparison of the prognostic value of MUST, ECOG-PS, mGPS and CT derived body composition analysis in patients with advanced lung cancer. Clin Nutr ESPEN 40:349–356. https://doi.org/10.1016/j.clnesp.2020.08.003
Tierney JF, Stewart LA, Ghersi D et al (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8(16):1745–6215. https://doi.org/10.1186/1745-6215-8-16
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605. https://doi.org/10.1007/s10654-010-9491-z
Relli V, Trerotola M, Guerra E et al (2019) Abandoning the Notion of Non-Small Cell Lung Cancer. Trends Mol Med 25(7):585–594. https://doi.org/10.1016/j.molmed.2019.04.012
Beadsmoore CJ, Screaton NJ (2003) Classification, staging and prognosis of lung cancer. Eur J Radiol 45(1):8–17. https://doi.org/10.1016/s0720-048x(02)00287-5
Molina JR, Yang P, Cassivi SD et al (2008) Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 83(5):584–594. https://doi.org/10.4065/83.5.584
Choi S, Park J (2020) Surgical outcomes and prognosis of non-small-cell lung cancer in patients with chronic lung diseases: a retrospective analysis. Eur J Cardiothorac Surg 58(2):357–364. https://doi.org/10.1093/ejcts/ezaa060
Clausen MM, Langer SW (2019) Improving the prognosis for lung cancer patients. Acta Oncol 58(8):1077–1078. https://doi.org/10.1080/0284186X.2019.1632477
Aleixo GFP, Shachar SS, Nyrop KA et al (2020) Myosteatosis and prognosis in cancer: systematic review and meta-analysis. Crit Rev Oncol Hematol 145:102839. https://doi.org/10.1016/j.critrevonc.2019.102839
Ahn H, Kim DW, Ko Y et al (2021) Updated systematic review and meta-analysis on diagnostic issues and the prognostic impact of myosteatosis: a new paradigm beyond sarcopenia. Ageing Res Rev 70:101398. https://doi.org/10.1016/j.arr.2021.101398
Miljkovic I, Cauley JA, Wang PY et al (2013) Abdominal myosteatosis is independently associated with hyperinsulinemia and insulin resistance among older men without diabetes. Obesity 21(10):2118–2125. https://doi.org/10.1002/oby.20346
Kovalik JP, Slentz D, Stevens RD et al (2011) Metabolic remodeling of human skeletal myocytes by cocultured adipocytes depends on the lipolytic state of the system. Diabetes 60(7):1882–1893. https://doi.org/10.2337/db10-0427
Dev R, Bruera E, Dalal S (2018) Insulin resistance and body composition in cancer patients. Ann Oncol 29(2):18–26. https://doi.org/10.1093/annonc/mdx815
Souza NC, Gonzalez MC, Martucci RB et al (2020) Frailty is associated with myosteatosis in obese patients with colorectal cancer. Clin Nutr 39(2):484–491. https://doi.org/10.1016/j.clnu.2019.02.026
Dodson S, Baracos VE, Jatoi A et al (2011) Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annu Rev Med 62:265–279. https://doi.org/10.1146/annurev-med-061509-131248
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420(6917):860–867. https://doi.org/10.1038/nature01322
Yamashita S, Iwahashi Y, Miyai H et al (2020) Myosteatosis as a novel prognostic biomarker after radical cystectomy for bladder cancer. Sci Rep 10(1):22146. https://doi.org/10.1038/s41598-020-79340-9
Miljkovic I, Vella CA, Allison M (2021) Computed tomography-derived myosteatosis and metabolic disorders. Diabetes Metab J 45(4):482–491. https://doi.org/10.4093/dmj.2020.0277
Xiao J, Caan BJ, Weltzien E et al (2018) Associations of pre-existing co-morbidities with skeletal muscle mass and radiodensity in patients with non-metastatic colorectal cancer. J Cachexia Sarcopenia Muscle 9(4):654–663. https://doi.org/10.1002/jcsm.12301
Kim EY, Kim YS, Park I et al (2015) Prognostic significance of CT-determined sarcopenia in patients with small-cell lung cancer. J Thorac Oncol 10(12):1795–1799. https://doi.org/10.1097/jto.0000000000000690
Yang M, Shen Y, Tan L et al (2019) Prognostic value of sarcopenia in lung cancer: a systematic review and meta-analysis. Chest 156(1):101–111. https://doi.org/10.1016/j.chest.2019.04.115
Pamoukdjian F, Bouillet T, Lévy V et al (2018) Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: a systematic review. Clin Nutr 37(4):1101–1113. https://doi.org/10.1016/j.clnu.2017.07.010
Stretch C, Aubin JM, Mickiewicz B et al (2018) Sarcopenia and myosteatosis are accompanied by distinct biological profiles in patients with pancreatic and periampullary adenocarcinomas. PLoS ONE 13(5):e0196235. https://doi.org/10.1371/journal.pone.0196235
Funding
This study was funded by Nanjing Medical science and technology development Foundation (Grant number ZKX17024).
Author information
Authors and Affiliations
Contributions
SF performed the study, analyzed the dada, and wrote the manuscript. HM and RH collected the data. YL and JZ carried out additional data analysis. ZZ and YZ contributed to the study design and were responsible for the revision and submission of the manuscript. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Informed consent
For this type of study, formal consent is not required.
Consent to participate
Not applicable.
Consent for publication
All the authors consent to the publication of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Feng, S., Mu, H., Hou, R. et al. Prognostic value of myosteatosis in patients with lung cancer: a systematic review and meta-analysis. Int J Clin Oncol 27, 1127–1138 (2022). https://doi.org/10.1007/s10147-022-02181-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-022-02181-1