Abstract
Background
Although the prognosis of patients experiencing recurrences after surgery for pancreatic cancer is extremely poor, patients who develop recurrence in the lung have a better prognosis compared to other types of recurrence. We performed a histo-immunological analysis of the metastatic specimens to identify specific features of this patient subgroup.
Methods
We performed immunohistochemistry for CD4+, CD8+, CD45RO+, Foxp3, and PD-L1 in the lung (n = 22), peritoneal (n = 18), and liver (n = 6) metastases of pancreatic cancer. As microenvironmental and immunonutritional investigations, the tumor-stroma ratio and prognostic nutritional index (PNI) were utilized in the integrative analysis of immunological features.
Results
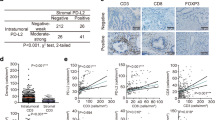
We identified significantly increased tumor-infiltrating CD4+, CD8+, and CD45RO+ cells in lung metastasis, compared with peritoneal and liver metastases (lung vs. peritoneum/liver, CD4: P < 0.001/P = 0.015, CD8: P < 0.001/P = 0.038, CD45RO: P = 0.022/P = 0.012). The CD8/Foxp3 ratio was higher in the lung than in the liver (P = 0.024). PD-L1 expression was significantly higher in lung metastasis than in peritoneal metastasis (P = 0.010). Furthermore, we found that lung metastasis had fewer cancer stroma than peritoneal metastasis (P < 0.001). A higher PNI was observed in patients with lung metastasis, and PNI was positively correlated with tumor-infiltrating lymphocytes in metastatic sites.
Conclusion
We identified that lung metastasis revealed an immunologically “hot” tumor with increased TILs and PD-L1 expression. This specific feature suggests that patients with lung metastasis can be candidates for immunotherapy, such as immune checkpoint inhibitors; therefore, our study provides a framework for developing individualized treatment strategies for this patient subgroup.







Similar content being viewed by others
References
Siegel RL, Miller KD, Fuchs HE et al (2021) Cancer Statistics, 2021. CA Cancer J Clin 71(1):7–33. https://doi.org/10.3322/caac.21654
Groot VP, Gemenetzis G, Blair AB et al (2018) Defining and predicting early recurrence in 957 patients with resected pancreatic ductal adenocarcinoma. Ann Surg. https://doi.org/10.1097/SLA.0000000000002734
Rahib L, Smith BD, Aizenberg R et al (2014) Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Can Res 74(11):2913–2921. https://doi.org/10.1158/0008-5472.CAN-14-0155
McGuigan A, Kelly P, Turkington RC et al (2018) Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol 24(43):4846–4861. https://doi.org/10.3748/wjg.v24.i43.4846
Mizrahi JD, Surana R, Valle JW et al (2020) Pancreatic cancer. Lancet 395(10242):2008–2020. https://doi.org/10.1016/S0140-6736(20)30974-0
Groot VP, Rezaee N, Wu W et al (2018) Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg 267(5):936–945. https://doi.org/10.1097/SLA.0000000000002234
Jones RP, Psarelli EE, Jackson R et al (2019) Patterns of recurrence after resection of pancreatic ductal adenocarcinoma: a secondary analysis of the ESPAC-4 randomized adjuvant chemotherapy trial. JAMA Surg 154(11):1038–1048. https://doi.org/10.1001/jamasurg.2019.3337
Kruger S, Haas M, Burger PJ et al (2016) Isolated pulmonary metastases define a favorable subgroup in metastatic pancreatic cancer. Pancreatology 16(4):593–598. https://doi.org/10.1016/j.pan.2016.03.016
Yamashita K, Miyamoto A, Hama N et al (2015) Survival impact of pulmonary metastasis as recurrence of pancreatic ductal adenocarcinoma. Dig Surg 32(6):464–471. https://doi.org/10.1159/000439545
Zheng B, Ohuchida K, Yan Z et al (2017) Primary recurrence in the lung is related to favorable prognosis in patients with pancreatic cancer and postoperative recurrence. World J Surg 41(11):2858–2866. https://doi.org/10.1007/s00268-017-4068-6
Yasukawa M, Kawaguchi T, Kawai N et al (2017) Surgical treatment for pulmonary metastasis of pancreatic ductal adenocarcinoma: study of 12 cases. Anticancer Res 37(10):5573–5576. https://doi.org/10.21873/anticanres.11990
Arnaoutakis GJ, Rangachari D, Laheru DA et al (2011) Pulmonary resection for isolated pancreatic adenocarcinoma metastasis: an analysis of outcomes and survival. J Gastrointest Surg 15(9):1611–1617. https://doi.org/10.1007/s11605-011-1605-8
Pan C, Liu H, Robins E et al (2020) Next-generation immuno-oncology agents: current momentum shifts in cancer immunotherapy. J Hematol Oncol 13(1):29. https://doi.org/10.1186/s13045-020-00862-w
Tempero MA, Malafa MP, Al-Hawary M et al (2021) Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 19(4):439–457. https://doi.org/10.6004/jnccn.2021.0017
Maleki Vareki S (2018) High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. J Immunother Cancer 6(1):157. https://doi.org/10.1186/s40425-018-0479-7
Lloyd CM, Marsland BJ (2017) Lung homeostasis: influence of age, microbes, and the immune system. Immunity 46(4):549–561. https://doi.org/10.1016/j.immuni.2017.04.005
Shinto E, Hase K, Hashiguchi Y et al (2014) CD8+ and FOXP3+ tumor-infiltrating T cells before and after chemoradiotherapy for rectal cancer. Ann Surg Oncol 21(Suppl 3):S414-421. https://doi.org/10.1245/s10434-014-3584-y
Tavares MC, Sampaio CD, Lima GE et al (2021) A high CD8 to FOXP3 ratio in the tumor stroma and expression of PTEN in tumor cells are associated with improved survival in non-metastatic triple-negative breast carcinoma. BMC Cancer 21(1):901. https://doi.org/10.1186/s12885-021-08636-4
Zhu Y, Li M, Mu D et al (2016) CD8+/FOXP3+ ratio and PD-L1 expression associated with survival in pT3N0M0 stage esophageal squamous cell cancer. Oncotarget 7(44):71455–71465. https://doi.org/10.18632/oncotarget.12213
Paver EC, Cooper WA, Colebatch AJ et al (2021) Programmed death ligand-1 (PD-L1) as a predictive marker for immunotherapy in solid tumours: a guide to immunohistochemistry implementation and interpretation. Pathology 53(2):141–156. https://doi.org/10.1016/j.pathol.2020.10.007
Park E, Yoo JE, Hwang HK et al (2021) Combined tumor epithelial and stromal histopathology with keratin 81 expression predicts prognosis for pancreatic ductal adenocarcinoma. J Hepatobiliary Pancreat Sci. https://doi.org/10.1002/jhbp.1025
Sandberg TP, Stuart M, Oosting J et al (2019) Increased expression of cancer-associated fibroblast markers at the invasive front and its association with tumor-stroma ratio in colorectal cancer. BMC Cancer 19(1):284. https://doi.org/10.1186/s12885-019-5462-2
van Pelt GW, Kjaer-Frifeldt S, van Krieken J et al (2018) Scoring the tumor-stroma ratio in colon cancer: procedure and recommendations. Virchows Arch 473(4):405–412. https://doi.org/10.1007/s00428-018-2408-z
Okadome K, Baba Y, Yagi T et al (2020) Prognostic nutritional index, tumor-infiltrating lymphocytes, and prognosis in patients with esophageal cancer. Ann Surg 271(4):693–700. https://doi.org/10.1097/SLA.0000000000002985
Barrett RL, Pure E (2020) Cancer-associated fibroblasts and their influence on tumor immunity and immunotherapy. Elife. https://doi.org/10.7554/eLife.57243
Taube JM, Anders RA, Young GD et al (2012) Colocalization of inflammatory response with B7–h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 4(127):127ra137. https://doi.org/10.1126/scitranslmed.3003689
Bence C, Hofman V, Chamorey E et al (2020) Association of combined PD-L1 expression and tumour-infiltrating lymphocyte features with survival and treatment outcomes in patients with metastatic melanoma. J Eur Acad Dermatol Venereol 34(5):984–994. https://doi.org/10.1111/jdv.16016
Yu Y, Zeng D, Ou Q et al (2019) Association of survival and immune-related biomarkers with immunotherapy in patients with non-small cell lung cancer: a meta-analysis and individual patient-level analysis. JAMA Netw Open 2(7):e196879. https://doi.org/10.1001/jamanetworkopen.2019.6879
Author information
Authors and Affiliations
Contributions
Toshihide Sasaki: study concept and design; acquisition of clinical data; analysis and interpretation of data and statistical analysis; drafting of the manuscript. Satoshi Nishiwada: study concept and design; specimen providers; acquisition of clinical data; analysis and interpretation of data and statistical analysis; drafting of the manuscript. Kenji Nakagawa: specimen providers; acquisition of clinical data. Minako Nagai: specimen providers; acquisition of clinical data. Taichi Terai: specimen providers; acquisition of clinical data. Daisuke Hokuto: specimen providers; acquisition of clinical data. Satoshi Yasuda: specimen providers; acquisition of clinical data. Yasuko Matsuo: specimen providers; acquisition of clinical data. Shunsuke Doi: specimen providers; acquisition of clinical data. Masayuki Sho: study concept and design; specimen providers; acquisition of clinical data; drafting of the manuscript; revision of the article; final approval of the article. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10147_2022_2131_MOESM1_ESM.pdf
Supplementary Fig. 1 Cumulative recurrence rate after primary tumor resection in pancreatic cancer. Recurrence was significantly later in patients with lung metastasis than in patients with peritoneal, liver, and multi-site metastasis. ‡P < 0.01, §P < 0.001 (PDF 27 KB)
About this article
Cite this article
Sasaki, T., Nishiwada, S., Nakagawa, K. et al. Integrative analysis identifies activated anti-tumor immune microenvironment in lung metastasis of pancreatic cancer. Int J Clin Oncol 27, 948–957 (2022). https://doi.org/10.1007/s10147-022-02131-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-022-02131-x