Abstract
Purpose
Platinum-resistant ovarian cancer (PROC) is usually treated with single-agent chemotherapy. A synergistic effect of gemcitabine and platinum has been reported in PROC. We evaluated the efficacy and safety of gemcitabine and carboplatin with or without bevacizumab (GC ± B) in patients with PROC.
Methods
From April 2014 to April 2018, patients with PROC received gemcitabine on days 1 and 8, and carboplatin on day 1, with or without bevacizumab (Bev) on day 1 every 3 weeks. The primary endpoint was objective response rate (ORR). The secondary endpoints were disease control rate (DCR), progression-free survival (PFS), overall survival (OS), and rate of adverse events.
Results
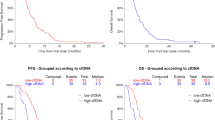
In total, 215 cycles were administered to 31 patients, of whom 21 received Bev and the median number of cycle for each patient was 6 (range, 2–19). The median platinum-free interval (PFI) was 4 months. The ORR and DCR were 51.9% and 92.6%, respectively. Median PFS and OS were 7.9 months and 16.1 months, respectively. PFS and OS of patients with 3–6 months PFI were significantly longer than those with PFI < 3 months (median PFS, 9.7 vs. 5.8 months; p < 0.01; median OS, 20.0 vs. 12.1 months; p = 0.03). Grade 3 or 4 hematological toxicities observed included neutropenia (71.0%), leukopenia (54.8%), anemia (51.6%), and thrombocytopenia (25.8%). No other grade 2–4 nonhematological toxicity was observed except for hypertension in one and CBDCA hypersensitivity reaction in two.
Conclusion
GC ± B may be effective and safe treatment alternative for PROC, especially with PFI of 3–6 months, despite hematological toxicity.



Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
National Cancer Registry (Ministry of Health, Labour and Welfare), tabulated by Cancer Information Service, National Cancer Center, Japan. https://ganjoho.jp/reg_stat/statistics/data/dl/en.html
Vital Statistics in Japan, tabulated by Cancer Information Service, National Cancer Center, Japan. https://ganjoho.jp/reg_stat/statistics/data/dl/en.html
Jayson GC, Kohn EC, Kitchener HC et al (2014) Ovarian cancer. Lancet 384:1376–1388. https://doi.org/10.1016/S0140-6736(13)62146-7
Pignata S, Cecere SC, Du Bois A et al (2017) Treatment of recurrent ovarian cancer. Ann Oncol 28:viii51–viii56. https://doi.org/10.1093/annonc/mdx441
Ushijima K (2010) Treatment for recurrent ovarian cancer—at first relapse. J Oncol. https://doi.org/10.1155/2010/497429
Mutch DG, Orlando M, Goss T et al (2007) Randomized phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer. J Clin Oncol 25:2811–2818. https://doi.org/10.1200/JCO.2006.09.6735
Gordon AN, Tonda M, Sun S et al (2004) Long-term survival advantage for women treated with pegylated liposomal doxorubicin compared with topotecan in a phase 3 randomized study of recurrent and refractory epithelial ovarian cancer. Gynecol Oncol 95:1–8. https://doi.org/10.1016/j.ygyno.2004.07.011
ten Bokkel HW, Lane SR, Ross GA (2004) Long-term survival in a phase III, randomised study of topotecan versus paclitaxel in advanced epithelial ovarian carcinoma. Ann Oncol 15:100–103. https://doi.org/10.1093/annonc/mdh025
Rose PG, Blessing JA, Mayer AR et al (1998) Prolonged oral etoposide as second-line therapy for platinum-resistant and platinum-sensitive ovarian carcinoma: a gynecologic oncology group study. J Clin Oncol 16:405–410. https://doi.org/10.1200/JCO.1998.16.2.405
Pujade-Lauraine E, Hilpert F, Weber B et al (2014) Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol 32:1302–1308. https://doi.org/10.1200/JCO.2013.51.4489
Flchtlnger-Schepman AMJ, Van Dijk-knijnenburg HCM, Van Der Velde-visser SD et al (1995) Cisplatin- and carboplatin-DNA adducts: is pt-ag the cytotoxic lesion? Carcinogenesis 16:2447–2453. https://doi.org/10.1093/carcin/16.10.2447
McHugh PJ, Spanswick VJ, Hartley JA (2001) Repair of DNA interstrand crosslinks: molecular mechanisms and clinical relevance. Lancet Oncol 2:483–490. https://doi.org/10.1016/S1470-2045(01)00454-5
Wynne P, Newton C, Ledermann JA et al (2007) Enhanced repair of DNA interstrand crosslinking in ovarian cancer cells from patients following treatment with platinum-based chemotherapy. Br J Cancer 97:927–933. https://doi.org/10.1038/sj.bjc.6603973
D’Agostino G, Amant F, Berteloot P et al (2003) Phase II study of gemcitabine in recurrent platinum-and paclitaxel-resistant ovarian cancer. Gynecol Oncol 88:266–269. https://doi.org/10.1016/S0090-8258(03)00011-8
Ledermann JA, Gabra H, Jayson GC et al (2010) Inhibition of carboplatin-induced DNA interstrand cross-link repair by gemcitabine in patients receiving these drugs for platinum-resistant ovarian cancer. Clin Cancer Res 16:4899–4905. https://doi.org/10.1158/1078-0432.CCR-10-0832
Hansen MKG, Smerdel MP, Waldstrøm M et al (2020) Carboplatin re-treatment in platinum-resistant epithelial ovarian cancer patients. Cancer Chemother Pharmacol 86:751–759. https://doi.org/10.1007/s00280-020-04162-5
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
John G, Rustin S, Vergote I et al (2011) Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the gynecological cancer intergroup (GCIG). Int J Gynecol Cancer 21:419–423. https://doi.org/10.1097/IGC.0b013e3182070f17
Calvert AH, Newell DR, Gumbrell LA et al (1989) Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol 7:1748–1756. https://doi.org/10.1200/JCO.1989.7.11.1748
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. https://doi.org/10.1159/000180580
Aghajanian C, Blank SV, Goff BA et al (2012) OCEANS: A randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol 30:2039–2045. https://doi.org/10.1200/JCO.2012.42.0505
Greenwood M (1926) The natural duration of cancer. Reports on Public Health and Medical Subjects. London: Her Majesty's Stationery Office. 33:1–26
Ferrandina G, Ludovisi M, Lorusso D et al (2008) Phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in progressive or recurrent ovarian cancer. J Clin Oncol 26:890–896. https://doi.org/10.1200/JCO.2007.13.6606
Brewer CA, Blessing JA, Nagourney RA et al (2006) Cisplatin plus gemcitabine in platinum-refractory ovarian or primary peritoneal cancer: a phase II study of the Gynecologic Oncology Group. Gynecol Oncol 103:446–450. https://doi.org/10.1016/j.ygyno.2006.03.018
Sharma R, Graham J, Mitchell H et al (2009) Extended weekly dose-dense paclitaxel/carboplatin is feasible and active in heavily pre-treated platinum-resistant recurrent ovarian cancer. Br J Cancer 100:707–712. https://doi.org/10.1038/sj.bjc.6604914
Lortholary A, Largillier R, Weber B et al (2012) Weekly paclitaxel as a single agent or in combination with carboplatin or weekly topotecan in patients with resistant ovarian cancer: the CARTAXHY randomized phase II trial from groupe d’investigateurs nationaux pour l’etude des cancers ovariens (GINECO). Ann Oncol 23:346–352. https://doi.org/10.1093/annonc/mdr149
Oncologico I, Buda A, Floriani I et al (2004) Randomised controlled trial comparing single agent paclitaxel vs epidoxorubicin plus paclitaxel in patients with advanced ovarian cancer in early progression after platinum-based chemotherapy: an Italian collaborative study from the “Mario Negri” Institut. Br J Cancer 90:2112–2117. https://doi.org/10.1038/sj.bjc.6601787
Sehouli J, Stengel D, Oskay-oezcelik G et al (2008) Nonplatinum topotecan combinations versus topotecan alone for recurrent ovarian cancer: results of a phase III study of the North-Eastern German Society of Gynecological Oncology Ovarian Cancer Study Group. J Clin Oncol 26:3176–3182. https://doi.org/10.1200/JCO.2007.15.1258
Takasaki K, Miyamoto M, Takano M et al (2018) Addition of bevacizumab to gemcitabine for platinum-resistant recurrent ovarian cancer: a retrospective analysis. Cancer Chemother Pharmacol 81:809–814. https://doi.org/10.1007/s00280-018-3552-5
Nagao S, Kogiku A, Suzuki K et al (2020) A phase II study of the combination chemotherapy of bevacizumab and gemcitabine in women with platinum-resistant recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Ovarian Res 13:1–6. https://doi.org/10.1186/s13048-020-0617-y
Takase N, Matsumoto K, Onoe T et al (2015) 4-Step 4-h carboplatin desensitization protocol for patients with gynecological malignancies showing platinum hypersensitivity: a retrospective study. Int J Clin Oncol 20:566–573. https://doi.org/10.1007/s10147-014-0731-1
Inayama Y, Hamanishi J, Matsumura N et al (2018) Antitumor effect of nivolumab on subsequent chemotherapy for platinum-resistant ovarian cancer. Oncologist 23:1382–1384. https://doi.org/10.1634/theoncologist.2018-0167
Hamanishi J, Mandai M, Ikeda T et al (2015) Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol 33:4015–4022. https://doi.org/10.1200/JCO.2015.62.3397
Matulonis UA, Santin AD, Lisyanskaya AS et al (2019) Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol 30:1080–1087. https://doi.org/10.1093/annonc/mdz135
Liu JF, Herold C, Gray KP et al (2019) Assessment of combined nivolumab and bevacizumab in relapsed ovarian cancer: a phase 2 clinical trial. JAMA Oncol 5:1731–1738. https://doi.org/10.1001/jamaoncol.2019.3343
Domchek SM, Aghajanian C, Shapira-frommer R et al (2016) Efficacy and safety of olaparib monotherapy in germline BRCA1/2 mutation carriers with advanced ovarian cancer and three or more lines of prior therapy. Gynecol Oncol 140:199–203. https://doi.org/10.1016/j.ygyno.2015.12.020
Pfisterer J, Shannon CM, Baumann K et al (2020) Bevacizumab and platinum-based combinations for recurrent ovarian cancer: a randomised, open-label, phase 3 trial. Lancet Oncol 21:699–709. https://doi.org/10.1016/S1470-2045(20)30142-X
Ito K, Nakagawa M, Hori K et al (2020) 834P A phase II study of gemcitabine, cisplatin, and bevacizumab for first recurrent and refractory ovarian clear-cell carcinoma (KCOG-G1601 trial). Ann Oncol 31:S627. https://doi.org/10.1016/j.annonc.2020.08.973
Poveda AM, Selle F, Hilpert F et al (2015) Bevacizumab combined with weekly paclitaxel, pegylated liposomal doxorubicin, or topotecan in platinum-resistant recurrent ovarian cancer: analysis by chemotherapy cohort of the randomized phase III aurelia trial. J Clin Oncol 33:3836–3838. https://doi.org/10.1200/JCO.2015.63.1408
Acknowledgements
We thank Editage (www.editage.com) for English language editing.
Funding
No funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Nasu, H., Nishio, S., Park, J. et al. Platinum rechallenge treatment using gemcitabine plus carboplatin with or without bevacizumab for platinum-resistant ovarian cancer. Int J Clin Oncol 27, 790–801 (2022). https://doi.org/10.1007/s10147-021-02103-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-021-02103-7