Abstract
Background
More than 50% children with high-risk neuroblastoma (HR-NBL) experience disease progression, which we hypothesise is due to non-response of primary tumour to treatment. Current imaging techniques are unable to characterise response in primary tumour (necrotic versus viable tissue) at diagnosis or follow-up.
Objectives
Compare clinico-histological characteristics between primary 123ImIBG-avid tumours that became entirely 123ImIBG-non-avid (responders) after induction chemotherapy (IC) versus primary 123ImIBG-avid tumour that remained 123ImIBG-avid (non-responders).
Methods
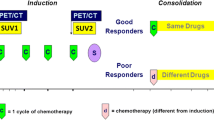
Retrospective review of clinico-radiological data of children diagnosed with 123ImIBG-avid HR-NBL at our centre (2005–2016). Patients received Rapid COJEC IC and two additional courses of TVD if metastatic response was inadequate. Primary tumour 123ImIBG response was assessed qualitatively as positive, negative or intermediate at diagnosis and after IC. Post-surgical histopathology slices were marked considering percentage of viable tissue.
Results
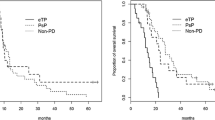
Sixteen of 61 patients showed complete primary tumour 123ImIBG response, 20 partial response, while 25 no response. There was no statistically significant difference between clinical demographics of complete responders and group of non- or partial responders. Mean percentage of viable tumour cells was higher in non-responders than in complete responders (44.6% vs 20.6%; p = 0.05). Five-year EFS was significantly higher in complete responders than non-responders (43 ± 15% vs 7 ± 6%; p < 0.005).
Conclusions
123ImIBG response in primary HR-NBL correlates with amount of necrotic tissue, skeletal metastatic 123ImIBG response and outcome. An entirely 123ImIBG non-avid tumour can still harbour viable tumour cells. Therefore, our findings do not support utility of primary tumour 123ImIBG response in decision making regarding residual tumour surgery. Combining both, primary and metastatic 123ImIBG response will improve interpretability of clinical trial results.



Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
Code availability
Not applicable.
References
Maris JM (2010) Recent advances in neuroblastoma. N Engl J Med 362:2202–2211
Yanik GA, Parisi MT, Shulkin BL et al (2013) Semiquantitative mIBG scoring as a prognostic indicator in patients with stage 4 neuroblastoma: a report from the Children’s oncology group. J Nucl Med 54:541–548
Decarolis B, Schneider C, Hero B et al (2013) Iodine-123 metaiodobenzylguanidine scintigraphy scoring allows prediction of outcome in patients with stage 4 neuroblastoma: results of the Cologne interscore comparison study. J Clin Oncol 31:944–951
Lewington V, Lambert B, Poetschger U et al (2017) 123I-mIBG scintigraphy in neuroblastoma: development of a SIOPEN semi-quantitative reporting, method by an international panel. Eur J Nucl Med Mol Imaging 44:234–241
Hishiki T, Horie H, Higashimoto Y et al (2014) Histological features of primary tumors after induction or high-dose chemotherapy in high-risk neuroblastoma. Pediatr Surg Int 30:919–926
Bomken S, Davies B, Chong L et al (2011) Percentage tumor necrosis following chemotherapy in neuroblastoma correlates with MYCN status but not survival. Pediatr Hematol Oncol 28:106–114
George RE, Variend S, Cullinane C et al (2001) Relationship between histopathological features, MYCN amplification, and prognosis: a UKCCSG study. United Kingdom Children Cancer Study Group. Med Pediatr Oncol 36:169–176
Ladenstein R, Lambert B, Pötschger U et al (2018) Validation of the mIBG skeletal SIOPEN scoring method in two independent high-risk neuroblastoma populations: the SIOPEN/HR-NBL1 and COG-A3973 trials. Eur J Nucl Med Mol Imaging 45:292–305
Katzenstein HM, Cohn SL, Shore RM et al (2004) MACROBUTTON HTMLDirect Scintigraphic response by 123I-metaiodobenzylguanidine scan correlates with event-free survival in high-risk neuroblastoma. J Clin Oncol 22:3909–3915
Ladenstein R, Valteau-Couanet D, Brock P et al (2010) Randomized trial of prophylactic granulocyte colony-stimulating factor during rapid COJEC induction in pediatric patients with high-risk neuroblastoma: the European HR-NBL1/SIOPEN study. J Clin Oncol 28(21):3516–3524
Bagatell R, McHugh K, Naranjo A et al (2016) Assessment of primary site response in children with high-risk neuroblastoma: an international multicenter study. J Clin Oncol 34:740–746
Dataset for histopathological reporting of peripheral neuroblastic tumours. https://www.rcpath.org/uploads/assets/8a8d8d30-fc7e-4e3f-a2be4663901547bc/G104-Dataset-for-histopathological-reporting-of-peripheral-neuroblastic-tumours.pdf. Accessed May 2019
Bombardieri E, Giammarile F, Aktolun C et al (2010) MACROBUTTON HTMLDirect 131I/123I metaiodobenzylguanidine (mIBG) scintigraphy: procedure guidelines for tumour imaging. Eur J Nucl Med Mol Imaging 37:2436–2446
Park JR, Bagatell R, Cohn SL et al (2017) Revisions to the international neuroblastoma response criteria: a consensus statement from the National Cancer Institute clinical trials planning meeting. J Clin Oncol 35:2580–2587
Matthay KK, Shulkin B, Ladenstein R et al (2010) Criteria for evaluation of disease extent by (123)I-metaiodobenzylguanidine scans in neuroblastoma: a report for the International Neuroblastoma Risk Group (INRG) Task Force. Br J Cancer 102(9):1319–1326
Matthay KK, Edeline V, Lumbroso J et al (2003) Correlation of early metastatic response by 123I-metaiodobenzylguanidine scintigraphy with overall response and event-free survival in stage IV neuroblastoma. J Clin Oncol 21:2486–2491
Cohn SL, Pearson AD, London WB et al (2009) INRG Task Force. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol 27:289–297
Evans AE, D’Angio GJ (2005) Age at diagnosis and prognosis in children with neuroblastoma. J Clin Oncol 23:6443–6444
Brodeur GM, Pritchard J, Berthold F et al (1994) Revisions of the international criteria for neuroblastoma diagnosis, staging and response to treatment. Prog Clin Biol Res 385:363–369
Marachelian A, Shimada H, Sano H et al (2012) The significance of serial histopathology in a residual mass for outcome of intermediate risk stage 3 neuroblastoma. Pediatr Blood Cancer 58:675–681
Miyauchi J, Matsuoka K, Oka T et al (1997) Histopathologic findings of advanced neuroblastoma after intensive induction chemotherapy. J Pediatr Surg 32:1620–1623
Tsuchida Y, Miyauchi J, Kuroiwa M et al (2005) Histologic survey of neuroblastomas after intensive induction chemotherapy. Pediatr Blood Cancer 45:656–662
Du L, Liu L, Zhang C et al (2014) Role of surgery in the treatment of patients with high-risk neuroblastoma who have a poor response to induction chemotherapy. J Pediatr Surg 49(4):528–533
Funding
Dr Elwira Szychot was a recipient of the National Institute for Health Research award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflict of interest to disclose.
Ethical approval
Approval for this study was obtained from the Research and Development Office of Great Ormond Street Hospital for Children NHS Foundation Trust (Number 15DC07) in London, United Kingdom, and was compliant with the 1964 Helsinki Declaration and its later amendments.
Consent to participate
Informed consent from parents/guardians was obtained for the experimental nature of the treatment and results publication.
Consent for publication
Verbal consent from parents/guardians was obtained for publishing the results of our study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Szychot, E., Morgenstern, D., Chopra, M. et al. Clinical impact of primary tumour 123ImIBG response to induction chemotherapy in children with high-risk neuroblastoma. Int J Clin Oncol 27, 253–261 (2022). https://doi.org/10.1007/s10147-021-02039-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-021-02039-y