Abstract
Objective
The aim of this study was to evaluate the clinical significance of the on-treatment C-reactive protein (CRP) status during systemic treatment as the predictive marker for the response of subsequent nivolumab monotherapy in patients with refractory metastatic renal cell carcinoma (mRCC).
Patients and methods
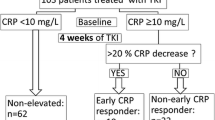
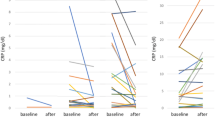
A total of 73 mRCC patients treated with nivolumab were retrospectively reviewed. We evaluated the serum CRP levels before and after molecular-targeted treatments. Patients whose CRP did not exceed baseline value were defined as the CRP-control group and the others were defined as the CRP-progression group. The clinical impact of CRP-control on the efficacy of nivolumab was assessed.
Results
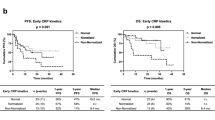
Twenty-four patients (33%) were categorized into the CRP-control group. The CRP-control group patients (median PFS not reached) had significantly longer PFS than the CRP-progression group (median PFS 11.9 months, 95% confidence interval, CI 4.1–19.8, p = 0.038). The CRP-control group had a tendency of longer OS from nivolumab initiation than the CRP-progression group (p = 0.071). By multivariate analysis, the on-treatment CRP-control was the independent predictive factor for PFS (hazard ratio HR 0.37, 95% CI 0.14–0.99, p = 0.047).
Conclusion
The on-treatment CRP-control could be the predictive factor for the efficacy of nivolumab in refractory mRCC patients.

Similar content being viewed by others
References
Motzer RJ, Escudier B, McDermott DF et al (2015) Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1803–1813
Ishihara H, Kondo T, Takagi T et al (2018) Immediate progressive disease in patients with metastatic renal cell carcinoma treated with nivolumab: a multi-institution retrospective study. Target Oncol 13:611–619
Buder-Bakhaya K, Hassel JC (2018) Biomarkers for clinical benefit of immune checkpoint inhibitor treatment-a review from the melanoma perspective and beyond. Front Immunol 9:1474
Zeng F, Wei H, Yeoh E et al (2016) Inflammatory markers of CRP, IL6, TNFalpha, and soluble TNFR2 and the risk of ovarian cancer: a meta-analysis of prospective studies. Cancer Epidemiol Biomarkers Prev 25:1231–1239
Zhou B, Shu B, Yang J et al (2014) C-reactive protein, interleukin-6 and the risk of colorectal cancer: a meta-analysis. Cancer Causes Control 25:1397–1405
Beuselinck B, Vano YA, Oudard S et al (2014) Prognostic impact of baseline serum C-reactive protein in patients with metastatic renal cell carcinoma (RCC) treated with sunitinib. BJU Int 114:81–89
Takamatsu K, Mizuno R, Omura M et al (2018) Prognostic value of baseline serum C-reactive protein level in intermediate-risk group patients with metastatic renal-cell carcinoma treated by first-line vascular endothelial growth factor-targeted therapy. Clin Genitourin Cancer 16(4):e927–e933
Takamatsu K, Mizuno R, Tanaka N et al (2019) Prognostic value of serum C-reactive protein level prior to second-line treatment in intermediate risk metastatic renal cell carcinoma patients. Int J Clin Oncol 24:1069–1074
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (Version 1.1). Eur J Cancer 45:228–247
Heng DY, Xie W, Regan MM et al (2009) Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 27:5794–5799
Motzer RJ, Hutson TE, Tomczak P et al (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356:115–124
Hudes G, Carducci M, Tomczak P et al (2007) Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 356:2271–2281
Motzer RJ, Hutson TE, Cella D et al (2013) Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 369:722–731
Takagi T, Kondo T, Kennoki T et al (2013) Comparison of survival rates in patients with metastatic renal cell carcinoma according to treatment era including cytokine and targeted therapy. Jpn J Clin Oncol 43:439–443
Escudier B, Sharma P, McDermott DF et al (2017) CheckMate 025 randomized phase 3 study: outcomes by key baseline factors and prior therapy for nivolumab versus everolimus in advanced renal cell carcinoma. Eur Urol 72(6):962–971
Motzer RJ, Tannir NM, McDermott DF et al (2018) Nivolumab plus Ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 378:1277–1290
Kondo T, Nomura M, Otsuka A et al (2018) Predicting marker for early progression in unresectable melanoma treated with nivolumab. Int J Clin Oncol 24(2):323–327
Zahoor H, Barata PC, Jia X et al (2018) Patterns, predictors and subsequent outcomes of disease progression in metastatic renal cell carcinoma patients treated with nivolumab. J Immunother Cancer 6:107
Sproston NR, Ashworth JJ (2018) Role of C-reactive protein at sites of inflammation and infection. Front Immunol 9:754
Alberti L, Thomachot MC, Bachelot T et al (2004) IL-6 as an intracrine growth factor for renal carcinoma cell lines. Int J Cancer 111:653–661
Oya M, Horiguchi A, Mizuno R et al (2003) Increased activation of CCAAT/enhancer binding protein-beta correlates with the invasiveness of renal cell carcinoma. Clin Cancer Res 9:1021–1027
Huber V, Camisaschi C, Berzi A et al (2017) Cancer acidity: an ultimate frontier of tumor immune escape and a novel target of immunomodulation. Semin Cancer Biol 43:74–89
Topalian SL, Hodi FS, Brahmer JR et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454
Chan LC, Li CW, Xia W et al (2019) IL-6/JAK1 pathway drives PD-L1 Y112 phosphorylation to promote cancer immune evasion. J Clin Invest 129:3324–3338
Snyder A, Makarov V, Merghoub T et al (2014) Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 371:2189–2199
McDermott DF, Huseni MA, Atkins MB et al (2018) Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med 24:749–757
Tumeh PC, Harview CL, Yearley JH et al (2014) PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515:568–571
Acknowledgements
Supported in part by grants-in-aid from the Ministry of Education, Science, Sports, and Culture of Japan (20K09585 to R.M. and 19K16807 to K.T.)
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
Ryuichi Mizuno MD has received honoraria from Bristol, Novartis, and Pfizer. Mototsugu Oya MD has received honoraria from Bayer, Bristol, Novartis, and Pfizer. Other authors declare that they have no conflict of interest.
Ethical approval
Ethical approval was waived by the local Ethics Committee of Keio University in view of the retrospective nature of the study. All procedures performed in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Because this was a retrospective cohort study, informed consent was waived. Opt out was done on the web site of Keio University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Takamatsu, K., Mizuno, R., Baba, Y. et al. On-treatment C-reactive protein control could predict response to subsequent anti-PD-1 treatment in metastatic renal cell carcinoma. Int J Clin Oncol 26, 1500–1505 (2021). https://doi.org/10.1007/s10147-021-01930-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-021-01930-y