Abstract
Background
We assessed the efficacy and safety of bevacizumab and S-1 chemotherapy for patients with previously treated advanced non-squamous non-small-cell lung cancer (NSCLC).
Methods
This was a prospective single-arm study, including patients with non-squamous NSCLC who had received at least one chemotherapy regimen along with a platinum-based regimen. Bevacizumab 15 mg/kg was intravenously administered every 3 weeks, and S-1 40 mg/m2 was orally administered twice daily from day 1 (evening) through day 15 (morning). The treatment continued for 3 weeks/cycle until disease progression or until unacceptable toxicities occurred. During the lead-in part, six patients were evaluated for dose-limiting toxicity (DLT) rate. In phase II, the primary endpoint was objective response rate (ORR). Secondary endpoints were progression-free survival (PFS), overall survival (OS), and safety.
Results
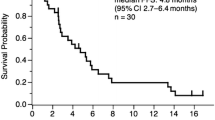
In the lead-in part, we evaluated the safety in the first six patients and observed no DLT. In phase II, a total of 46 patients were enrolled from September 2012 to December 2018. The median follow-up duration was 13.7 months [95% confidence interval (CI) 1.4–72.0]. The ORR was 28.3%. The median PFS and OS were 4.3 (95% CI 2.9–5.9) and 15.0 months (95% CI 9.8–30.3), respectively. The most common adverse events were hypertension (65.2%), diarrhea (47.8%), mucositis oral (45.7%), and proteinuria (43.5%), and the most common grade 3 adverse events were hypertension (23.9%) and proteinuria (6.5%). Grade 4/5 adverse events were not observed.
Conclusion
Bevacizumab and S-1 combination chemotherapy showed high activity and were well tolerated in patients with previously treated advanced non-squamous NSCLC.

Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics. CA Cancer J Clin 67(1):7–30. https://doi.org/10.3322/caac.21387
Fossella FV, DeVore R, Kerr RN et al (2000) Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol 18(12):2354–2362. https://doi.org/10.1200/jco.2000.18.12.2354
Shepherd FA, Dancey J, Ramlau R et al (2000) Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 18(10):2095–2103. https://doi.org/10.1200/jco.2000.18.10.2095
Nokihara H, Lu S, Mok TSK et al (2017) Randomized controlled trial of S-1 versus docetaxel in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy (East Asia S-1 Trial in Lung Cancer). Ann Oncol 28(11):2698–2706. https://doi.org/10.1093/annonc/mdx419
Sandler A, Gray R, Perry MC et al (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355(24):2542–2550. https://doi.org/10.1056/NEJMoa061884
Garon EB, Ciuleanu T-E, Arrieta O et al (2014) Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. The Lancet 384(9944):665–673. https://doi.org/10.1016/s0140-6736(14)60845-x
Nukatsuka M, Saito H, Nakagawa F et al (2012) Combination therapy using oral S-1 and targeted agents against human tumor xenografts in nude mice. Exp Ther Med 3(5):755–762. https://doi.org/10.3892/etm.2012.484
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10(1):1–10. https://doi.org/10.1016/0197-2456(89)90015-9
Totani Y, Saito Y, Hayashi M et al (2009) A phase II study of S-1 monotherapy as second-line treatment for advanced non-small cell lung cancer. Cancer Chemother Pharmacol 64(6):1181–1185. https://doi.org/10.1007/s00280-009-0981-1
Hanna N, Shepherd FA, Fossella FV et al (2004) Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 22(9):1589–1597. https://doi.org/10.1200/JCO.2004.08.163
Scagliotti GV, Parikh P, von Pawel J et al (2008) Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 26(21):3543–3551. https://doi.org/10.1200/JCO.2007.15.0375
Gandhi L, Rodriguez-Abreu D, Gadgeel S et al (2018) Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 378(22):2078–2092. https://doi.org/10.1056/NEJMoa1801005
Okamoto I, Yoshioka H, Morita S et al (2010) Phase III trial comparing oral S-1 plus carboplatin with paclitaxel plus carboplatin in chemotherapy-naive patients with advanced non-small-cell lung cancer: results of a west Japan oncology group study. J Clin Oncol 28(36):5240–5246. https://doi.org/10.1200/JCO.2010.31.0326
Kubota K, Sakai H, Katakami N et al (2015) A randomized phase III trial of oral S-1 plus cisplatin versus docetaxel plus cisplatin in Japanese patients with advanced non-small-cell lung cancer: TCOG0701 CATS trial. Ann Oncol 26(7):1401–1408. https://doi.org/10.1093/annonc/mdv190
Liu W, Zhang J, Yao X et al (2018) Bevacizumab-enhanced antitumor effect of 5-fluorouracil via upregulation of thymidine phosphorylase through vascular endothelial growth factor A/vascular endothelial growth factor receptor 2-specificity protein 1 pathway. Cancer Sci 109(10):3294–3304. https://doi.org/10.1111/cas.13779
Nishino K, Imamura F, Kumagai T et al (2015) A randomized phase II study of bevacizumab in combination with docetaxel or S-1 in patients with non-squamous non-small-cell lung cancer previously treated with platinum based chemotherapy (HANSHIN Oncology Group 0110). Lung Cancer 89(2):146–153. https://doi.org/10.1016/j.lungcan.2015.05.022
Yamada K, Ichiki M, Takahashi K et al (2016) A multicenter phase II trial of S-1 combined with bevacizumab after platinum-based chemotherapy in patients with advanced non-squamous non-small cell lung cancer. Cancer Chemother Pharmacol 78(3):501–507. https://doi.org/10.1007/s00280-016-3101-z
Nishijima-Futami Y, Minami S, Futami S et al (2017) Phase II study of S-1 plus bevacizumab combination therapy for patients previously treated for non-squamous non-small cell lung cancer. Cancer Chemother Pharmacol 79(6):1215–1220. https://doi.org/10.1007/s00280-017-3321-x
Ohyanagi F, Yanagitani N, Kudo K et al (2014) Phase II study of docetaxel-plus-bevacizumab combination therapy in patients previously treated for advanced non-squamous non-small cell lung cancer. Anticancer Res 34(9):5153–5158
Acknowledgements
The authors thank all the study participants who provided clinical data for the analysis.
Author information
Authors and Affiliations
Contributions
KK, FO and MN contributed to the study concept and design and all authors approved it. TH, NY, and MN were involved in statistical analysis of the patient data and writing of the article. TH, NY, FO, KK, AH, YT, SN, RA, KU, SK, and MN were involved in patient clinical data collection. TH wrote the first draft of the manuscript, and all authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Makoto Nishio reports honoraria from Ono Pharmaceutical, Bristol-Myers Squibb, Pfizer, Chugai Pharmaceutical, Eli Lilly, Taiho Pharmaceutical, AstraZeneca, Boehringer-ingelheim, MSD, and Novartis, research funding from Novartis, Daiichi Sankyo, Taiho Pharmaceutical, Bristol-Myers Squibb, Boehringer-ingelheim, Ono Pharmaceutical, Eli Lilly, Chugai Pharmaceutical, AstraZeneca, Merck Sernon, MSD and Pfizer. Atsushi Horiike reports honoraria from Chugai Pharmaceutical and Taiho Pharmaceutical, research funding from Chugai Pharmaceutical. Noriko Yanagitani reports employment/leadership position/ advisory role of Chugai Pharmaceutical. Yuichi Tambo reports honoraria from AstraZeneca, Chugai Pharmaceutical, Taiho Pharmaceutical and MSD. Ken Uchibori reports employment/leadership position/ advisory role of Daiichi Sankyo. All the other authors have stated that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Hasegawa, T., Yanagitani, N., Ohyanagi, F. et al. Phase II study of the combination of S-1 with bevacizumab for patients with previously treated advanced non-squamous non-small-cell lung cancer. Int J Clin Oncol 26, 507–514 (2021). https://doi.org/10.1007/s10147-020-01822-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-020-01822-7