Abstract
Background
Fluorouracil and leucovorin combined with oxaliplatin or irinotecan plus bevacizumab (Bmab) or cetuximab (Cmab) are now widely accepted treatment options as first-line or second-line chemotherapy for metastatic colorectal cancer (mCRC). Sequential chemotherapy with oral 5-FU backbone for mCRC without using central venous ports is beneficial for both patients and physicians. We designed the SOBIC trial to validate the effectiveness of the first- and second-line oral combination chemotherapy for mCRC.
Patients and methods
From May 2010 through March 2013, 52 patients were enrolled from 47 institutions in the Hyogo Colorectal Cancer Surgery Group. First-line chemotherapy was S-1 + oxaliplatin (SOX) plus Bmab, and second-line chemotherapy after first-line failure was irinotecan + S-1 (IRIS) + Cmab, IRIS + Bmab, or IRIS based on the KRAS status.
Results
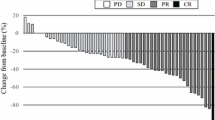
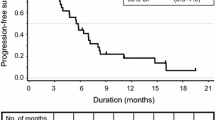
The 50 finally included patients received first-line chemotherapy. Second-line therapy was administered to 20 patients (40%): 12 patients received IRIS + Cmab and 8 patients received IRIS + Bmab. The median follow-up period was 48.6 months (range 35–67 months). The median second progression-free survival was 24.2 months (95% confidence interval [CI] 17.7–35.2). The response rate after first- and second-line chemotherapy was 46.7% and 15%, respectively. The median overall survival was 35.2 months (95% CI: 27.8 to not reached). The main grade 3–4 adverse events were sensory neuropathy (18%) and fatigue (10%). There were no treatment-related deaths.
Conclusion
Sequential S-1-based combination regimens including oxaliplatin, irinotecan, Bmab, and Cmab were beneficial for patients with mCRC.



Similar content being viewed by others
References
Yamazaki K, Nagase M, Tamagawa H et al (2016) Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (WJOG4407G). Ann Oncol 27:1539–1546
Satoh T, Sakata Y (2012) S-1 for the treatment of gastrointestinal cancer. Expert Opin Pharmacother 13:1943–1959
Yamada Y, Takahari D, Matsumoto H et al (2013) Leucovorin, fluorouracil, and oxaliplatin plus bevacizumab versus S-1 and oxaliplatin plus bevacizumab in patients with metastatic colorectal cancer (SOFT): an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol 14:1278–1286
Muro K, Boku N, Shimada Y et al (2010) Irinotecan plus S-1 (IRIS) versus fluorouracil and folinic acid plus irinotecan (FOLFIRI) as second-line chemotherapy for metastatic colorectal cancer: a randomised phase 2/3 noninferiority study (FIRIS study). Lancet Oncol 11:853–860
Schmoll HJ, Van Cutsem E, Stein A et al (2012) ESMO Consensus Guidelines for management of patients with colon and rectal cancer: a personalized approach to clinical decision making. Ann Oncol 23:2479–2516
Tournigand C, André T, Achille E et al (2004) FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 22:229–237
Fuchs CS, Marshall J, Mitchell E et al (2007) Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C study. J Clin Oncol 25:4779–4786
Cassidy J, Clarke S, Diaz-Rubio E et al (2008) Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol 26:2006–2012
Loupakis F, Cremolini C, Masi G et al (2014) Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 371:1609–1618
Heinemann V, von Weikersthal FL, Decker T et al (2014) FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 15:1065–1075
Venook AP, Niedzwiecki D, Innocenti F et al (2016) Impact of primary (1°) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol 34(Suppl 15):3504
Venook AP, Ou FS, Lenz HJ et al (2017) Impact of primary (1°) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): Analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol 35(Suppl 15):3503
Komatsu Y, Yuki S, Sogabe S et al (2012) Phase II study of combined chemotherapy with irinotecan and S-1 (IRIS) plus bevacizumab in patients with inoperable recurrent or advanced colorectal cancer. Acta Oncol 51:867–872
Minami H, Sai K, Saeki M et al (2007) Irinotecan pharmacokinetics/pharmacodynamics and UGT1A genetic polymorphisms in Japanese: roles of UGT1A1*6 and *28. Pharmacogenet Genomics 17:497–504
Acknowledgements
We thank all the patients, their families, the investigators, and medical staff. A list of participating institution: Department of Surgery, Meiwa Hospital; Department of Surgery, Kobe City Medical Center West Hospital; Department of Surgery, Japan Post Kyoto Teishin Hospital; Department of Surgery, Himeji St. Mary's Hospital; Department of Surgery, Kakogawa City Hospital; Department of Surgery, Sanda City Hospital; Department of Surgery, Kawanishi City Hospital; Division of Lower GI Surgery, Department of Surgery, Hyogo College of Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
N. Tomita has received research funding from Taiho Pharmaceutical Co., Ltd and Chugai Pharmaceutical Co., Ltd. All other authors have no conflicts of interest to declare.
Ethical approval
All procedures were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Nakamoto, Y., Noda, M., Mikami, R. et al. Phase II study of S-1-based sequential combination chemotherapy including oxaliplatin plus bevacizumab and irinotecan with or without cetuximab for metastatic colorectal cancer: the SOBIC trial. Int J Clin Oncol 25, 1285–1290 (2020). https://doi.org/10.1007/s10147-020-01657-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-020-01657-2