Abstract
Background
Liver resection is the most effective procedure for colorectal cancer liver metastasis (CRLM); however, early recurrence is an important problem that affects the postoperative prognoses of patients with CRLM. We previously suggested a therapeutic algorithm for CRLM using fluorodeoxyglucose-positron emission tomography (FDG-PET) and revealed the applicability of FDG-PET in predicting the prognosis after liver resection of CRLM. In this study, we assessed the correlation between FDG-PET and biological viability such as proliferation or metabolic activity.
Methods
We retrospectively evaluated 61 patients who underwent hepatectomy for CRLM. We assessed hypoxia inducible factor-1α (HIF-1α), pyruvate kinase isozyme M2 (PKM2), glucose transporter 1 (GLUT1), and Ki-67 expression via immunohistochemistry and evaluated the correlation between standardized uptake value (SUV) and these factors.
Results
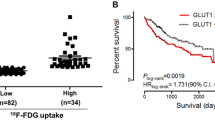
High HIF-1α, PKM2, and GLUT1 expression were positively correlated with high SUV expression (P = 0.0444, 0.0296, and 0.0245, respectively). Ki-67 and SUV were also positively correlated (P = 0.00164). HIF-1α expression and PKM2 expression were significantly correlated (P = 0.0430), and PKM2 expression and GLUT1 expression were extremely significantly correlated (P < 0.0001).
Conclusion
SUV reflected tumor proliferation or metabolic factors in CRLM. FDG-PET could be a useful modality for assessing tumor viability and may provide useful information regarding the appropriate treatment strategy for CRLM.



Similar content being viewed by others
References
Akgül Ö, Çetinkaya E, Ersöz Ş et al (2014) Role of surgery in colorectal cancer liver metastases. World J Gastroenterol 20:6113–6122
McNally SJ, Parks RW (2013) Surgery for colorectal liver metastases. Dig Surg 30:337–347
Allard MA, Adam R, Giuliante F et al (2017) Long-term outcomes of patients with 10 or more colorectal liver metastases. Br J Cancer 117:604–611
Simmonds PC, Primrose JN, Colquitt JL et al (2006) Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer 94:982–999
Fong Y, Fortner J, Sun RL et al (1999) Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 230:309–318 (discussion 318–321)
Hadden WJ, de Reuver PR, Brown K et al (2016) Resection of colorectal liver metastases and extra-hepatic disease: a systematic review and proportional meta-analysis of survival outcomes. HPB (Oxford) 18:209–220
Matias M, Casa-Nova M, Faria M et al (2015) Prognostic factors after liver resection for colorectal liver metastasis. Acta Med Port 28:357–369
Beppu T, Sakamoto Y, Hasegawa K et al (2012) A nomogram predicting disease-free survival in patients with colorectal liver metastases treated with hepatic resection: multicenter data collection as a Project Study for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci 19:72–84
Angelsen JH, Viste A, Loes IM et al (2015) Predictive factors for time to recurrence, treatment and post-recurrence survival in patients with initially resected colorectal liver metastases. World J Surg Oncol 13:328
Nagashima I, Takada T, Adachi M et al (2006) Proposal of criteria to select candidates with colorectal liver metastases for hepatic resection: comparison of our scoring system to the positive number of risk factors. World J Gastroenterol 12:6305–6309
Wu Y, Li C, Zhao J et al (2016) Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios predict chemotherapy outcomes and prognosis in patients with colorectal cancer and synchronous liver metastasis. World J Surg Oncol 14:289
Willowson KP, Hayes AR, Chan DLH et al (2017) Clinical and imaging-based prognostic factors in radioembolisation of liver metastases from colorectal cancer: a retrospective exploratory analysis. EJNMMI Res 7:46
Watanabe A, Harimoto N, Araki K et al (2018) A new strategy based on fluorodeoxyglucose-positron emission tomography for managing liver metastasis from colorectal cancer. J Surg Oncol 118:1088–1095
Kato H, Kuwano H, Nakajima M et al (2002) Comparison between positron emission tomography and computed tomography in the use of the assessment of esophageal carcinoma. Cancer 94:921–928
Takahashi S, Kuroki Y, Nasu K et al (2006) Positron emission tomography with F-18 fluorodeoxyglucose in evaluating colorectal hepatic metastasis down-staged by chemotherapy. Anticancer Res 26:4705–4711
Moulton CA, Gu CS, Law CH et al (2014) Effect of PET before liver resection on surgical management for colorectal adenocarcinoma metastases: a randomized clinical trial. JAMA 311:1863–1869
Muralidharan V, Kwok M, Lee ST et al (2012) Prognostic ability of 18F-FDG PET/CT in the assessment of colorectal liver metastases. J Nucl Med 53:1345–1351
Lee HS, Kim HO, Hong YS et al (2014) Prognostic value of metabolic parameters in patients with synchronous colorectal cancer liver metastasis following curative-intent colorectal and hepatic surgery. J Nucl Med 55:582–589
Riedl CC, Akhurst T, Larson S et al (2007) 18F-FDG PET scanning correlates with tissue markers of poor prognosis and predicts mortality for patients after liver resection for colorectal metastases. J Nucl Med 48:771–775
Binderup T, Knigge UP, Federspiel B et al (2013) Gene expression of glucose transporter 1 (GLUT1), hexokinase 1 and hexokinase 2 in gastroenteropancreatic neuroendocrine tumors: correlation with F-18-fluorodeoxyglucose positron emission tomography and cellular proliferation. Diagnostics (Basel) 3:372–384
Sato J, Kitagawa Y, Yamazaki Y et al (2013) 18F-fluoromisonidazole PET uptake is correlated with hypoxia-inducible factor-1alpha expression in oral squamous cell carcinoma. J Nucl Med 54:1060–1065
Strasberg SM (2005) Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg 12:351–355
Kanda Y (2013) Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 48:452–458
Chun YS, Vauthey JN, Boonsirikamchai P et al (2009) Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA 302:2338–2344
Shindoh J, Loyer EM, Kopetz S et al (2012) Optimal morphologic response to preoperative chemotherapy: an alternate outcome end point before resection of hepatic colorectal metastases. J Clin Oncol 30:4566–4572
Hosseini-Nik H, Fischer SE, Moulton CA et al (2016) Diffusion-weighted and hepatobiliary phase gadoxetic acid-enhanced quantitative MR imaging for identification of complete pathologic response in colorectal liver metastases after preoperative chemotherapy. Abdom Radiol (NY) 41:231–238
Meng X, Li H, Kong L et al (2016) MRI In rectal cancer: correlations between MRI features and molecular markers Ki-67, HIF-1alpha, and VEGF. J Magn Reson Imaging 44:594–600
Deng SM, Zhang W, Zhang B et al (2015) Correlation between the uptake of 18F-fluorodeoxyglucose (18F-FDG) and the expression of proliferation-associated antigen Ki-67 in cancer patients: a meta-analysis. PLoS One 10:e0129028
Shannon AM, Bouchier-Hayes DJ, Condron CM et al (2003) Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat Rev 29:297–307
Bos R, van der Groep P, Greijer AE et al (2003) Levels of hypoxia-inducible factor-1alpha independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer 97:1573–1581
Schindl M, Schoppmann SF, Samonigg H et al (2002) Overexpression of hypoxia-inducible factor 1alpha is associated with an unfavorable prognosis in lymph node-positive breast cancer. Clin Cancer Res 8:1831–1837
Luo W, Hu H, Chang R et al (2011) Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 145:732–744
Ouyang Y, Li H, Bu J et al (2016) Hypoxia-inducible factor-1 expression predicts osteosarcoma patients' survival: a meta-analysis. Int J Biol Markers 31:e229–e234
Huang C, Huang Z, Bai P et al (2018) Expression of pyruvate kinase M2 in human bladder cancer and its correlation with clinical parameters and prognosis. Onco Targets Ther 11:2075–2082
Yang Y, Wu K, Liu Y et al (2017) Prognostic significance of metabolic enzyme pyruvate kinase M2 in breast cancer: a meta-analysis. Medicine (Baltimore) 96:e8690
Masoud GN, Li W (2015) HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B 5:378–389
Yang W, Zheng Y, Xia Y et al (2012) ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat Cell Biol 14:1295–1304
Acknowledgements
The authors thank Ms. Yukie Saito, Ms. Fumie Takada, Ms. Harumi Kanai, Ms. Tomoko Ubukata, Ms. Okada Aska, and Ms. Negishi Misato for their excellent assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Watanabe, A., Harimoto, N., Yokobori, T. et al. FDG-PET reflects tumor viability on SUV in colorectal cancer liver metastasis. Int J Clin Oncol 25, 322–329 (2020). https://doi.org/10.1007/s10147-019-01557-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-019-01557-0