Abstract
Background
The association between UGT1A1 polymorphism and etoposide-induced toxicities is still not clear. The aim of this study was to assess the association between uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) gene polymorphism and severe hematologic toxicities in Japanese patients receiving etoposide plus platinum chemotherapy for small-cell lung cancer.
Methods
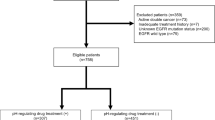
This retrospective analysis included patients with small-cell lung cancer who had received their first-line chemotherapy with etoposide plus cisplatin or carboplatin, between October 2008 and April 2018, at the University of Fukui Hospital. The relationship between UGT1A1 polymorphisms and first-cycle neutropenia as well as thrombocytopenia was evaluated.
Results
A total of 55 patients were enrolled. The incidence of grade 4 neutropenia during the first cycle of etoposide-based chemotherapy was higher in patients with homozygous (hmz) polymorphisms for UGT1A1*28 and *6 (*28/*28, *6/*6, and *6/*28) than in patients with wild-type (wt) (*1/*1) and heterozygous (htz) (*1/*28 and *1/*6) polymorphisms (88% vs 43% P = 0.03). The incidence of febrile neutropenia and grade 4 thrombocytopenia, however, was not significantly different. Multivariate analysis suggested that grade 4 neutropenia associated significantly with an hmz UGT1A1 genotype [odds ratio (OR) 11.3; P = 0.04] and administration of granulocyte colony-stimulating factor (G-CSF) before the neutrophil counts dropped to < 500 cells/µL (OR; P = 0.01).
Conclusions
UGT1A1*28 and UGT1A1*6 mutations might be regarded as predictors for etoposide-induced grade 4 neutropenia.
Similar content being viewed by others
References
O’Dwyer PJ, Leyland-Jones B, Alonso MT et al (1985) Etoposide (VP-16-213): current status of an active anticancer drug. N Engl J Med 312:692–700
Noda K, Nishiwaki Y, Kawahara M et al (2002) Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. New Engl J Med 346:85–91
Okamoto H, Watanabe K, Kunikane H et al (2007) Randomised phase III trial of carboplatin plus etoposide vs split doses of cisplatin plus etoposide in elderly or poor-risk patients with extensive disease small-cell lung cancer: JCOG 9702. Br J Cancer 97:162–169
Hryniuk W, Bush H (1984) The importance of dose intensity in chemotherapy of metastatic breast cancer. J Clin Oncol 2:1281–1288
Levin L, Hryniuk WM (1987) Dose intensity analysis of chemotherapy regimens in ovarian carcinoma. J Clin Oncol 5:756–767
Crawford J, Dale DC, Lyman GH (2004) Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer 100:228–237
Kuderer NM, Dale DC, Crawford J et al (2006) Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 106:2258–2266
Lyman GH, Michels SL, Reynolds MW et al (2010) Risk of mortality in patients with cancer who experience febrile neutropenia. Cancer 116:5555–5563
Fleming RA, Miller AA, Stewart CF (1989) Etoposide: an update. Clin Pharm 8:274–293
D’Incalci M, Rossi C, Zucchetti M et al (1986) Pharmacokinetics of etoposide in patients with abnormal renal and hepatic function. Cancer Res 46:2566–2571
Watanabe Y, Nakajima M, Ohashi N et al (2003) Glucuronidation of etoposide in human liver microsomes in specifically catalyzed by UDP-glucuronosyltransferase 1A1. Drug Metab Dispos 31:589–595
Wen Z, Tallman MN, Ali SY et al (2007) UDP-glucuronosyltransferase 1A1 is the principal enzyme responsible for etoposide glucuronidation in human liver and intestinal microsomes: structural characterization of phenolic and alcoholic glucuronides of etoposide and estimation of enzyme kinetics. Drug Metab Dispos 35:371–380
Bosma PJ, Chowdhury JR, Bakker C et al (1995) The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert’s syndrome. N Engl J Med 333:1171–1175
Takeuchi K, Kobayashi Y, Tamaki S et al (2004) Genetic polymorphisms of bilirubin uridine diphosphate-glucuronosyl transferase genein Japanese patients with Crigler–Najjar syndrome or Gilbert’s syndrome as well as in healthy Japanese subjects. J Gastroenterol Hepatol 19:1023–1028
Abumiya M, Takahashi N, Niioka T et al (2014) Influence of UGT1A1 6, 27, and 28 polymorphisms on nilotinib-induced hyperbilirubinemia in Japanese patients with chronic myeloid leukemia. Drug Metab Pharmacokinet 29:449–454
Meza-Junco J, Chu QS, Christensen O et al (2009) UGT1A1 polymorphism and hyperbilirubinemia in a patient who received sorafenib. Cancer Chemother Pharmacol 65:1–4
Xu CF, Reck BH, Xue Z et al (2010) Pazopanib-induced hyperbilirubinemia is associated with Gilbert’s syndrome UGT1A1 polymorphism. Br J Cancer 102:1371–1377
Rivory LP, Robert J (1995) Identification and kinetics of a beta-glucuronide metabolite of SN-38 in human plasma after administration of the camptothecin derivative irinotecan. Cancer Chemother Pharmacol 36:176–179
Haaz MC, Rivory L, Jantet S et al (1997) Glucuronidation of SN-38, the active metabolite of irinotecan, by human hepatic microsomes. Pharmacol Toxicol 80:91–96
Araki K, Fujita K, Ando Y et al (2006) Pharmacogenetic impact of polymorphisms in the coding region of the UGT1A1 gene on SN-38 glucuronidation in Japanese patients with cancer. Cancer Sci 97:1255–1259
Ando Y, Saka H, Ando M et al (2000) Polymorphisms of UDP-glucuronosyltransferase gene and irinotecan toxicity: a pharmacogenetic analysis. Cancer Res 60:6921–6926
Minami H, Sai K, Saeki M et al (2007) Irinotecan pharmacokinetics/pharmacodynamics and UGT1A genetic polymorphisms in Japanese: roles of UGT1A1*6 and *28. Pharmacogenet Genom 17:497–504
Hasegawa Y, Sarashina T, Ando M et al (2004) Rapid detection of UGT1A1 gene polymorphisms by newly developed Invader assay. Clin Chem 50:1479–1480
Satoh T, Ura T, Yamada Y et al (2011) Genotype-directed, dose-finding study of irinotecan in cancer patients with UGT1A1*28 and ⁄ or UGT1A1*6 polymorphisms. Cancer Sci 102:1868–1873
Japanese Society of Medical Oncology (2012) Practical guideline of febrile neutropenia (FN). Nankodo Co. Ltd., Tokyo
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Kanda Y (2013) Investigation of the freely-available easy-to-use software “EZR” (Easy R) for medical statistics. Bone Marrow Transplant 48:452–458
Kishi S, Yang W, Boureau B et al (2004) Effects of prednisone and genetic polymorphisms on etoposide disposition in children with acute lymphoblastic leukemia. Blood 103:67–72
Fukui T, Mitsufuji H, Kubota M et al (2011) Prevalence of topoisomerase I genetic mutations and UGT1A1 polymorphisms associated with irinotecan in individuals of Asian descent. Oncol Lett 2:923–928
Nishikawa Y, Kanai M, Narahara M et al (2017) Association between UGT1A1*28*28 genotype and lung cancer in the Japanese population. Int J Clin Oncol 22:269–273
Moriya H, Saito K, Helsby N et al (2013) The association between heterozygosity for UGT1A1*6. UGT1A1*28, and variation in the serum total-bilirubin level in healthy young Japanese adults. Genet Test Mol Biomark 17:464–469
Kringen MK, Piehler AP, Grimholt RM et al (2014) Serum bilirubin concentration in healthy adult North-Europeans is strictly controlled by the UGT1A1 TA-repeat variants. PLoS One 9:e90248. https://doi.org/10.1371/journal.pone.0090248
Hande KR, Wolff SN, Greco FA et al (1990) Etoposide kinetics in patients with obstructive jaundice. J Clin Oncol 8:1101–1107
Joel SP, Shah R, Clark PI et al (1996) Predicting etoposide toxicity: relationship to organ function and protein binding. J Clin Oncol 14:257–267
Mick R, Ratain MJ (1991) Modeling interpatient pharmacodynamic variability of etoposide. J Natl Cancer Inst 6:1560–1564
Acknowledgements
We would like to thank the participating patients for their contribution to this study. We would also like to thank Editage (http://www.editage.jp) for English language editing. The results in this manuscript have been partly presented at the 54th annual meeting of Japanese Society of Clinical Oncology, Oct. 21, 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
The need for informed consent was waived because of the retrospective nature of the study.
About this article
Cite this article
Negoro, Y., Yano, R., Yoshimura, M. et al. Influence of UGT1A1 polymorphism on etoposide plus platinum-induced neutropenia in Japanese patients with small-cell lung cancer. Int J Clin Oncol 24, 256–261 (2019). https://doi.org/10.1007/s10147-018-1358-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-018-1358-4