Abstract
Background
Neoadjuvant chemotherapy (NAC) involving two cycles of cisplatin plus fluorouracil is recommended in Japan as a standard treatment for resectable, locally advanced esophageal squamous cell carcinoma (ESCC). We have encountered patients who were administered incomplete chemotherapy because of adverse events or the patient’s refusal of treatment. Here, we retrospectively investigated the influence on perioperative outcomes and long-term prognosis of patients with ESCC who underwent complete (two cycles) or incomplete (one cycle) NAC.
Methods
We retrospectively investigated 133 patients with locally advanced ESCC of the thoracic esophagus who underwent NAC. We compared the perioperative results and prognoses of patients who underwent complete or incomplete NAC because of adverse events or the patient’s refusal of treatment.
Results
Of 133 patients, 37 patients did not receive the second cycle of NAC; the remaining 96 patients received the second cycle of NAC as scheduled. There were no significant differences in the clinical backgrounds, surgical results, or operative morbidity rates between the groups. Patients in both groups were similarly administered postoperative chemotherapy regimens. There was no significant difference in disease-free survival or overall survival.
Conclusions
We suggest that perioperative outcomes and long-term prognosis of patients with locally advanced ESCC were not significantly influenced, even if the patients did not receive a complete cycle of NAC. When certain adverse events occur after the first cycle of NAC, we believe that it is nevertheless possible to discontinue chemotherapy.
Similar content being viewed by others
Introduction
Esophageal cancer (EC) is the sixth leading cause of cancer-related mortality and the eighth most common cancer worldwide [1, 2]. The prognosis of patients with advanced EC is poor despite the development of multidisciplinary therapy [1, 2]. Neoadjuvant therapy followed by surgical resection is now the worldwide standard of care for resectable locally advanced EC [3]. Neoadjuvant chemoradiation therapy is commonly performed in Western countries [1, 3]. In contrast, in Japan, neoadjuvant chemotherapy (NAC) is the standard for resectable locally advanced EC according to the results of the Japan Clinical Oncology Group (JCOG) trial [4, 5]. NAC, which was adopted by our institution in 2008 for treating locally advanced esophageal squamous cell carcinoma (ESCC), has been recommended to patients since 2010. In principle, according to the results of the JCOG9907 trial [5], NAC involves two cycles of cisplatin plus fluorouracil (CF). However, some patients in our practice who receive the first cycle were unable to continue to the second cycle because of adverse events or the patient’s refusal of treatment. There have been no discussions, to our knowledge, asking whether incomplete NAC affects the prognosis of esophageal cancer.
Therefore, we retrospectively investigated the influence on perioperative outcomes and long-term prognosis of patients with ESCC who underwent complete (two cycles) or incomplete (one cycle) NAC.
Patients and methods
Inclusion criteria
This study involved consecutive patients who received NAC at Osaka City University Hospital (Osaka, Japan) from April 2008 to December 2014. The eligibility criteria were as follows: (a) resectable locally advanced thoracic EC, clinical stage IB–IV (excluding T4), according to the 7th edition of the Union for International Cancer Control (UICC) tumor–node–metastasis cancer staging system (UICC 7th) [6]; (b) histologically confirmed squamous cell carcinoma; (c) no active concomitant malignancy; and (d) an Eastern Cooperative Oncology Group performance status of 0–2, with adequate organ function. Computed tomography, endoscopic ultrasound, and positron emission tomography (if feasible) were used for staging. The Institutional Review Board of Osaka City University Hospital approved this study (Approval No. 3852).
Preoperative treatment
In principle, two cycles of NAC were scheduled. The CF regimen, which was generally chosen according to the results of the JCOG9907 trial [5], was administered as an intravenous infusion of cisplatin (80 mg/m2) on day 1 and a continuous infusion of fluorouracil (800 mg/m2) from days 1 to 5, with an interval of 4 weeks between the first day of each cycle. All patients underwent computed tomography to evaluate their responses to each cycle of NAC from days 15 to 21. The clinical response to chemotherapy was evaluated according to the Response Evaluation Criteria in Solid Tumors [7]. For patients with progressive disease (PD) after the first cycle of NAC, surgical resection was performed without the second cycle. For patients with stable disease (SD) or a partial response (PR) after the first cycle of NAC, the second cycle of NAC was scheduled, in principle. However, the second cycle was not administered to patients who developed adverse events or did not consent to undergo the next cycle. Surgical resection was scheduled 4–5 weeks after completing NAC.
Data collection and perioperative analysis
Clinical data were collected from the patient database of Osaka City University Hospital. We reviewed data on clinicopathological information, intraoperative factors, and postoperative morbidity and mortality after radical esophagectomy as well as the long-term outcomes of patients who did or did not undergo the second cycle of NAC. Tumor staging was determined using the 7th edition of the UICC tumor–node–metastasis cancer staging system [6]. All resected specimens were subjected to histopathological analysis. The pathologist classified the histopathological grades of NAC according to the Japanese Classification of Esophageal Cancer, 11th edition [8]. Among postoperative complications, pneumonia was diagnosed if a patient had an infiltrate, revealed using chest imaging, with an associated fever or elevated white blood cell count. Recurrent laryngeal nerve paralysis was defined as a disturbance of vocal cord mobility determined using a flexible scope after extubation. Anastomotic leakage was defined as direct clinical observation of contrast extravasation. Chyle leakage was diagnosed by observations of a change in the quality of the pleural fluid from serous to milky or yellowish.
Statistical analysis
Patients’ data were analyzed using JMP 12 software (SAS Institute, Cary, NC, USA). Continuous variables were analyzed using the Mann–Whitney test. The Chi-square test was used to compare categorical variables between groups. The disease-free survival of postoperative patients without residual tumor (R0) was measured from the date of surgery to the date of the first evidence of disease. For patients who did not relapse, disease-free survival was censored at the last date on which the absence of relapse was confirmed. Overall survival was measured from the date of surgery to the date of death or last follow-up. Disease-free survival and overall curves were generated using the Kaplan–Meier method, and comparisons were made with the results of the log-rank test. p < 0.05 (two-sided) was considered to be statistically significant.
Results
Study design
The study design is illustrated in Fig. 1. After the first cycle of NAC, 26/159 patients developed PD; 24 patients underwent surgical resection and two underwent chemoradiation therapy without the second cycle of NAC. One hundred and thirty-three patients developed SD or a PR after the first cycle of NAC, and 37 patients did not receive the second cycle of NAC because of adverse events or the patient’s refusal of treatment (NAC1 group). The remaining 96 patients received the second cycle of NAC as scheduled (NAC2 group).
Patients’ characteristics
The NAC1 and NAC2 groups did not significantly differ in age, sex, or tumor location (Table 1). Although there was no difference in clinical stage between groups, many patients with advanced T categories were included in the NAC2 group. A contributing factor of M1 (distant metastasis) was metastasis in the supraclavicular lymph nodes or lymph nodes along the common hepatic artery. Patients in the NAC1 group had higher pretreatment creatinine levels compared with those in the NAC2 group. Representative adverse events after the first cycle of NAC of the NAC1 group were kidney injury and decreased neutrophil counts. In contrast, the NAC2 group mainly experienced gastrointestinal toxicity. However, laboratory data acquired immediately after definitive treatment (surgery or definitive chemoradiation therapy) showed that impaired renal function was more common in the NAC2 group (p < 0.05).
Operative and postoperative outcomes and postoperative treatment
Edematous and fibrous changes to tissues are often found in the mediastinal surgical field after NAC, and these changes make the procedure more difficult [9]. Despite the difference in the number of NAC cycles, the surgical outcomes did not significantly differ between groups (Table 2). The main reasons for conversion from thoracoscopic surgery to open thoracotomy were severe pleural adhesions or contiguous organ invasion by the tumor. Eleven patients in the NAC2 group underwent noncurative resection (R1/R2) because of direct tumor invasion of a surrounding organ (pericardial membrane [T4a], [n = 1]; aorta or trachea [T4b], [n = 10]). The groups did not significantly differ in the overall frequency of postoperative complications or length of hospitalization. There were no significant differences between groups in the chemotherapy grade in response to NAC. Further, of the 93 patients in the NAC2 group, 19 had a chemotherapy grade of 0.
For patients with a pathological node-positive status (pN-positive) who recovered adequate organ function and requested postoperative chemotherapy, adjuvant chemotherapy was administered after obtaining their informed consent. Thus, we administered adjuvant chemotherapy to less than half of patients who underwent curative resection. For patients who did not sufficiently respond to the CF regimen of NAC, a taxane-based regimen (paclitaxel or docetaxel) was additionally administered. For patients who had severe kidney injury or hematological toxicity after NAC, a reduced dose (20–50%) of each agent was administered. All patients who underwent noncurative resection (R1/R2) received definitive chemoradiation therapy after surgery.
TNM staging after treatment
The contributing factor of the final extent of M1 was metastasis in the supraclavicular lymph nodes or lymph nodes along the common hepatic artery. The NAC2 group included more patients with ypT4 (ypStage IIIC) than the NAC1 group. Therefore, the proportion of patients with advanced disease was higher in the NAC2 group (Table 3). However, both groups were down-staged after NAC (Fig. 2).
Long-term outcomes
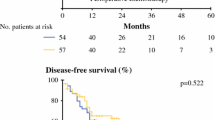
The median follow-up of censored patients was 39 months (range 1–111 months). The frequency and pattern of relapses did not significantly differ between groups (Table 4). The disease-free survival curves revealed that the patterns of relapses between groups were not significantly different (p = 0.213) (Fig. 3), and there was no significant difference in overall survival (Fig. 4) between groups (p = 0.676). As noted above, long-term outcomes of the groups were not significantly different.
Discussion
In the present study, 37/159 patients (23.3%) did not receive the second cycle of NAC because of adverse events or the patient’s refusal of treatment. We observed little influence on perioperative outcomes and long-term prognosis of patients who did not undergo the scheduled two cycles of NAC. However, there is a possibility of selection bias, because the attending physician tended to administer the second cycle to patients with advanced disease. Randomized controlled studies of NAC for esophageal cancer typically involve multicycle (two or three cycles) chemotherapy regimens [5, 10,11,12,13,14]. In these studies, the second cycle was not administered to allow for the possibility of future curative resection of patients who experienced PD during the first cycle. In contrast, the second or subsequent cycle was conducted as scheduled for patients who had SD or PD during the first cycle. These studies evaluated the effectiveness of treatment, including patients who did not complete chemotherapy.
Here, we encountered some patients who did not undergo the scheduled cycles of chemotherapy because of adverse events or the patients’ refusal of treatment. In randomized controlled trials [10,11,12,13,14], 6–32% of the patients receiving NAC did not complete the scheduled full cycles. The major reasons for incomplete NAC were the patient’s refusal of treatment and adverse events such as hematological toxicity and kidney injury, other than PD. Preservation of renal function is a critical issue. In the NAC1 group, many patients had an acute kidney injury after the first cycle of NAC. We considered that patients in the NAC1 group had potentially impaired renal function before treatment (Table 1). However, laboratory data acquired immediately just after definitive treatment showed that impaired renal function was more common in the NAC2 group (p < 0.05). Patients in the NAC1 group may recover faster from renal dysfunction. We anticipate that adverse events or patients’ refusal of treatment increase in accordance with the number of cycles of NAC. Therefore, we believe that it is important to consider the risk of unfavorable perioperative outcomes caused by prolonged adverse events after chemotherapy, because radical esophagectomy for esophageal cancer is a highly advanced, invasive operation with potentially high morbidity and mortality [15, 16]. In contrast, we assume that efficacy will increase with repeating cycles of NAC. The influence on the long-term prognosis of patients with incomplete NAC is unclear. In the present study, there were no significant differences in short-term or long-term outcomes of these patients.
The advantages of NAC include down-staging, improving the curative resection (R0) rate, completing the protocol [5], and confirming chemosensitivity. The histological response to NAC correlates with the prognosis of EC [17, 18]. By undergoing even one cycle of chemotherapy, the chemosensitivity to a given chemotherapy regimen can be confirmed. Further, we were able to choose the optimal regimen of postoperative chemotherapy according to the histological response to NAC. In the present study, there was no significant difference in the histological responses to NAC between the two groups. It was reported that the response to chemotherapy in the second cycle decreased compared with that to the first cycle [19]. There is a possibility that a better histological response would not be achieved even if we repeated the NAC cycle.
The effectiveness of adjuvant chemotherapy for postoperative patients with EC who receive NAC is unclear. We do not recommend adjuvant chemotherapy to patients with a pathological node-negative status (pN0), according to the results of the JCOG9204 trial [20]. However, if patients with pN-positive disease desired postoperative chemotherapy, adjuvant chemotherapy was conducted with informed consent. Consequently, we administered adjuvant chemotherapy to approximately 70% of patients who were pN-positive in both groups. However, we believe that future clinical trials should be conducted to better assess the value of adjuvant therapy after NAC. For example, a clinical trial using docetaxel, cisplatin plus 5-FU (DCF) as preoperative therapy is ongoing in Japan (JCOG1109) [21]. Using a more intense chemotherapy regimen may cause patients to discontinue chemotherapy, which is intrinsically invasive. Our present results suggest that it is possible to safely discontinue chemotherapy to prepare for subsequent surgery when adverse events occur after the first cycle of NAC.
Conclusions
Perioperative outcomes and long-term prognosis of our patients with locally advanced ESCC were not significantly influenced, even if they were unable to undergo a complete cycle of NAC. We suggest therefore that chemotherapy can be discontinued to prepare for subsequent surgery for patients who experience adverse events. However, a prospective cohort study is required to validate this conclusion.
References
Lagergren J, Smyth E, Cunningham D et al (2017) Oesophageal cancer. Lancet. https://doi.org/10.1016/S0140-6736(17)31462-9
Cools-Lartigue J, Spicer J, Ferri LE (2015) Current status of management of malignant disease: current management of esophageal cancer. J Gastrointest Surg 19:964–972
Rustgi AK, El-Serag HB (2014) Esophageal carcinoma. N Engl J Med 371:2499–2509
Sohda M, Kuwano H (2017) Current status and future prospects for esophageal cancer treatment. Ann Thorac Cardiovasc Surg 23:1–11
Ando N, Kato H, Igaki H et al (2012) A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 19:68–74
Sobin LH, Gospodarowicz MK, Wittekind C (2010) TNM classification of malignant tumors, 7th edn. Wiley-Blackwell, Oxford
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Japan Esophageal Society (2017) Japanese classification of esophageal cancer, 11th edition: part II and III. Esophagus 14: 37–65
Fujiwara Y, Lee S, Kishida S et al (2017) Safety and feasibility of thoracoscopic esophagectomy after neoadjuvant chemotherapy for esophageal cancer. Surg Today 47:1356–1360
Law S, Fok M, Chow S, Chu KM et al (1997) Preoperative chemotherapy versus surgical therapy alone for squamous cell carcinoma of the esophagus: a prospective randomized trial. J Thorac Cardiovasc Surg 114:210–217
Kelsen DP, Ginsberg R, Pajak TF et al (1998) Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med 339:1979–1984
Ancona E, Ruol A, Santi S et al (2001) Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long term survival of patients with resectable esophageal squamous cell carcinoma: final report of a randomized, controlled trial of preoperative chemotherapy versus surgery alone. Cancer 91:2165–2174
Medical Research Council Oesophageal Cancer Working Group (2002) Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 359: 1727–1733
Boonstra JJ, Kok TC, Wijnhoven BP et al (2011) Chemotherapy followed by surgery versus surgery alone in patients with resectable oesophageal squamous cell carcinoma: long-term results of a randomized controlled trial. BMC Cancer 11:181
Giwa F, Salami A, Abioye AI (2018) Hospital esophagectomy volume and postoperative length of stay: a systematic review and meta-analysis. Am J Surg 215:155–162
Li KK, Wang YJ, Liu XH et al (2017) The effect of postoperative complications on survival of patients after minimally invasive esophagectomy for esophageal cancer. Surg Endosc 31:3475–3482
Shimada Y, Watanabe G, Yamasaki S et al (2000) Histological response of cisplatin predicts patients’ survival in oesophageal cancer and p53 protein accumulation in pretreatment biopsy is associated with cisplatin sensitivity. Eur J Cancer 36:987–993
Schneider PM, Baldus SE, Metzger R et al (2005) Histomorphologic tumor regression and lymph node metastases determine prognosis following neoadjuvant radiochemotherapy for esophageal cancer: implications for response classification. Ann Surg 242:684–692
Motoori M, Yano M, Yasuda T et al (2013) Early response to neoadjuvant chemotherapy in advanced esophageal cancer evaluated by computed tomography predicts the utility of a second cycle of chemotherapy. Mol Clin Oncol 1:521–526
Ando N, Iizuka T, Ide H et al (2003) Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study–JCOG9204. J Clin Oncol 21:4592–4596
Nakamura K, Kato K, Igaki H, Japan Esophageal Oncology Group/Japan Clinical Oncology Group et al (2013) Three-arm phase III trial comparing cisplatin plus 5-FU (CF) versus docetaxel, cisplatin plus 5-FU (DCF) versus radiotherapy with CF (CF-RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT study). Jpn J Clin Oncol 43:752–755
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
About this article
Cite this article
Fujiwara, Y., Lee, S., Kishida, S. et al. Influence of incomplete neoadjuvant chemotherapy on esophageal carcinoma. Int J Clin Oncol 23, 877–885 (2018). https://doi.org/10.1007/s10147-018-1291-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-018-1291-6