Abstract
Background
Introducing a new surgical technology may affect behaviors and attitudes of patients and surgeons about clinical practice. Robot-assisted laparoscopic radical prostatectomy (RALP) was approved in 2012 in Japan. We investigated whether the introduction of this system affected the treatment of organ-confined prostate cancer (PCa) and the use of radical prostatectomy (RP).
Methods
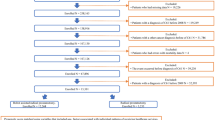
We conducted a retrospective multicenter study on 718 patients with clinically determined organ-confined PCa treated at one of three Japanese academic institutions in 2011 (n = 338) or 2013 (n = 380). Two patient groups formed according to the treatment year were compared regarding the clinical characteristics of PCa, whether referred or screened at our hospital, comorbidities and surgical risk, and choice of primary treatment.
Results
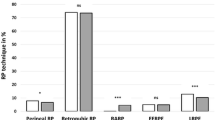
Distribution of PCa risk was not changed by the introduction of RALP. Use of RP increased by 70% (from 127 to 221 cases, p < 0.0001), whereas the number of those undergoing radiotherapy or androgen deprivation therapy decreased irrespective of the disease risk of PCa. Increased use of RP (from 34 to 100 cases) for intermediate- or high-risk PCa patients with mild perioperative risk (American Society of Anesthesiologists score 2) accounted for 70% of the total RP increase, whereas the number of low- or very low-risk PCa patients with high comorbidity scores (Charlson Index ≥ 4) increased from 8 to 25 cases, accounting for 18%. Use of expectant management (active surveillance, watchful waiting) in very low-risk PCa patients was 15% in 2011 and 12% in 2013 (p = 0.791).
Conclusions
Introduction of a robotic surgical system had little effect on the risk distribution of PCa. Use of RP increased, apparently due to increased indications in patients who are candidates for RP but have mild perioperative risk. Although small, there was an increase in the number of RPs performed on patients with severe comorbidities but with low-risk or very low-risk PCa.




Similar content being viewed by others
References
GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. IARC Publications 2012
Gray PJ, Lin CC, Cooperberg MR et al (2017) Temporal trends and the impact of race, insurance, and socioeconomic status in the management of localized prostate cancer. Eur Urol 71:729–737
Neuner JM, See WA, Pezzin LE et al (2012) The association of robotic surgical technology and hospital prostatectomy volumes: increasing market share through the adoption of technology. Cancer 118:371–377
Miller AB (2007) Commentary: implications of the frequent occurrence of occult carcinoma of the prostate. Int J Epidemiol 36:282–284
Yaxley JW, Coughlin GD, Chambers SK et al (2016) Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: early outcomes from a randomised controlled phase 3 study. Lancet 388:1057–1066
Barbash GI, Glied SA (2010) New technology and health care costs—the case of robot-assisted surgery. N Engl J Med 363:701–704
Sugihara T, Yasunaga H, Horiguchi H et al (2014) Robot-assisted versus other types of radical prostatectomy: population-based safety and cost comparison in Japan, 2012–2013. Cancer Sci 105:1421–1426
Jacobs BL, Zhang Y, Schroeck FR et al (2013) Use of advanced treatment technologies among men at low risk of dying from prostate cancer. JAMA 309:2587–2595
National Comprehensive Cancer Network Practice Guidelines in Oncology. 2017. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed 1 Aug 2017
Charlson ME, Pompei P, Ales KL et al (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
ASA Physical Status Classification System. 2014. https://www.asahq.org/resources/clinical-information/asa-physical-status-classification-system. Accessed 1 Aug 2017
van As NJ, Norman AR, Thomas K et al (2008) Predicting the probability of deferred radical treatment for localised prostate cancer managed by active surveillance. Eur Urol 54:1297–1305
Soloway MS, Soloway CT, Eldefrawy A et al (2010) Careful selection and close monitoring of low-risk prostate cancer patients on active surveillance minimizes the need for treatment. Eur Urol 58:831–835
Klotz L, Zhang L, Lam A et al (2010) Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol 28:126–131
van den Bergh RC, Roemeling S, Roobol MJ et al (2009) Outcomes of men with screen-detected prostate cancer eligible for active surveillance who were managed expectantly. Eur Urol 55:1–8
Tosoian JJ, Trock BJ, Landis P et al (2011) Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol 29:2185–2190
Kamidono S, Ohshima S, Hirao Y et al (2008) Evidence-based clinical practice Guidelines for Prostate Cancer (Summary—JUA 2006 Edition). Int J Urol 15:1–18
Bul M, Zhu X, Valdagni R et al (2013) Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol 63:597–603
Kakehi Y, Kamoto T, Shiraishi T et al (2008) Prospective evaluation of selection criteria for active surveillance in Japanese patients with stage T1cN0M0 prostate cancer. Jpn J Clin Oncol 38:122–128
Schroeck FR, Kaufman SR, Jacobs BL et al (2013) Technology diffusion and diagnostic testing for prostate cancer. J Urol 190:1715–1720
Jemal A, Fedewa SA, Ma J et al (2015) Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA 314:2054–2061
Fleshner K, Carlsson SV, Roobol MJ (2017) The effect of the USPSTF PSA screening recommendation on prostate cancer incidence patterns in the USA. Nat Rev Urol 14:26–37
Tsukamoto T, Tanaka S (2015) Robot-assisted laparoscopic radical prostatectomy for patients with prostatic cancer and factors promoting installation of the robotic surgical equipment-questionnaire survey. Hinyokika Kiyo 61:321–328
Novara G, Ficarra V, Rosen RC et al (2012) Systematic review and meta-analysis of perioperative outcomes and complications after robot-assisted radical prostatectomy. Eur Urol 62:431–452
Takizawa I, Ohori M, Ohno Y et al (2016) Pathologic and prognostic outcomes of very low- and low-risk prostate cancer according to the National Comprehensive Cancer Network Guidelines in Japanese Patients with Radical Prostatectomy. J Cancer Ther 7:239–246
Sugihara T, Yasunaga H, Matsui H et al (2017) Accessibility to surgical robot technology and prostate-cancer patient behavior for prostatectomy. Jpn J Clin Oncol 47(7):647–651
Tsukamoto T, Tanaka S (2016) Have case loads of radical surgery for prostate cancer been concentrated in hospitals with robotic equipment?—analyses with questionnaire survey and diagnostic procedure combination (DPC) data. Hinyokika Kiyo 62:179–185
Acknowledgements
This study was supported in part by a grant-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (to O.O., #26253078). The authors allow the journal to review our data if requested.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No author has any conflict of interest.
About this article
Cite this article
Kobayashi, T., Kanao, K., Araki, M. et al. Impact of a robotic surgical system on treatment choice for men with clinically organ-confined prostate cancer. Int J Clin Oncol 23, 347–352 (2018). https://doi.org/10.1007/s10147-017-1203-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-017-1203-1