Abstract
Objectives
The purpose of this phase II study was to explore the efficacy and safety of an alternating regimen consisting of folinic acid, 5-fluorouracil (5-FU) and oxaliplatin (mFOLFOX6) plus bevacizumab, and folinic acid, 5-FU and irinotecan (FOLFIRI) plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer.
Methods
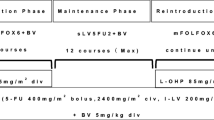
Fifty-two patients with metastatic colorectal cancer received an alternating regimen consisting of four cycles of mFOLFOX6 plus bevacizumab followed by four cycles of FOLFIRI plus bevacizumab until disease progression. The primary endpoint was progression-free survival.
Results
The median age was 60 years (range 37–75 years). Median progression-free survival was 14.2 months (95 % confidence interval [CI] 10.6–16.3) and median overall survival was 28.4 months (95 % CI 22.6–39.1). The overall response rate was 60.0 % (95 % CI 45.2–73.6). Regarding toxicity, the commonest grade 3–4 hematological adverse events were neutropenia (34.6 %) and leukopenia (7.7 %), and the commonest grade 3–4 non-hematological adverse events were anorexia (13.5 %), fatigue (9.6 %), nausea (9.6 %), and vomiting (9.6 %). Bevacizumab-related grade 3–4 adverse events included hypertension (1.9 %) and thrombosis (1.9 %).
Conclusions
An alternating regimen consisting of mFOLFOX6 plus bevacizumab and FOLFIRI plus bevacizumab is an effective and well-tolerated first-line chemotherapy combination for patients with metastatic colorectal cancer.


Similar content being viewed by others
References
Jemal A, Tiwari RC, Murray T et al (2004) Cancer statistics, 2004. CA Cancer J Clin 54:8–29
Parkin DM (2001) Global cancer statistics in the year 2000. Lancet Oncol 2:533–543
Fuchs CS, Marshall J, Mitchell E et al (2007) Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C study. J Clin Oncol 25:4779–4786
Tournigand C, Andre T, Achille E et al (2004) FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 22:229–237
Cassidy J, Clarke S, Diaz-Rubio E et al (2008) Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol 26:2006–2012
Hurwitz H, Fehrenbacher L, Novotny W et al (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342
Van Cutsem E, Kohne CH, Hitre E et al (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360:1408–1417
Grothey A, Sargent D, Goldberg RM et al (2004) Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol 22:1209–1214
Grothey A, Sargent D (2005) Overall survival of patients with advanced colorectal cancer correlates with availability of fluorouracil, irinotecan, and oxaliplatin regardless of whether doublet or single-agent therapy is used first line. J Clin Oncol 23:9441–9442
Falcone A, Ricci S, Brunetti I et al (2007) Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol 25:1670–1676
Souglakos J, Androulakis N, Syrigos K et al (2006) FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): a multicentre randomised phase III trial from the Hellenic Oncology Research Group (HORG). Br J Cancer 94:798–805
Masi G, Loupakis F, Salvatore L et al (2010) Bevacizumab with FOLFOXIRI (irinotecan, oxaliplatin, fluorouracil, and folinate) as first-line treatment for metastatic colorectal cancer: a phase 2 trial. Lancet Oncol 11:845–852
Montagnani F, Chiriatti A, Turrisi G et al (2011) A systematic review of FOLFOXIRI chemotherapy for the first-line treatment of metastatic colorectal cancer: improved efficacy at the cost of increased toxicity. Colorectal Dis 13:846–852
Oki E, Emi Y, Akagi Y et al (2013) Phase II trial of alternating mFOLFOX6 and FOLFIRI regimens in the first-line treatment for unresectable or metastatic colorectal cancer (KSCC0701). Oncology 84:233–239
Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9:669–676
Horita Y, Yamada Y, Kato K et al (2012) Phase II clinical trial of second-line FOLFIRI plus bevacizumab for patients with metastatic colorectal cancer: AVASIRI trial. Int J Clin Oncol 17:604–609
Bennouna J, Sastre J, Arnold D et al (2013) Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 14:29–37
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Trotti A, Colevas AD, Setser A et al (2003) CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 13:176–181
Tournigand C, Cervantes A, Figer A et al (2006) OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer—a GERCOR study. J Clin Oncol 24:394–400
Vaidyanathan G, Groman A, Wilding G et al (2010) Stop and go FOLFOX plus bevacizumab chemotherapy in the first-line treatment of metastatic colorectal cancer. Oncology 79:67–71
Aparicio J, Fernandez-Martos C, Vincent JM et al (2005) FOLFOX alternated with FOLFIRI as first-line chemotherapy for metastatic colorectal cancer. Clin Colorectal Cancer 5:263–267
Hebbar M, Tournigand C, Lledo G et al (2006) Phase II trial alternating FOLFOX-6 and FOLFIRI regimens in second-line therapy of patients with metastatic colorectal cancer (FIREFOX study). Cancer Invest 24:154–159
Heinemann V, von Weikersthal LF, Decker T et al (2014) FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 15:1065–1075
Yamada Y, Takahari D, Matsumoto H et al (2013) Leucovorin, fluorouracil, and oxaliplatin plus bevacizumab versus s-1 and oxaliplatin plus bevacizumab in patients with metastatic colorectal cancer (SOFT): an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol 14:1278–1286
Acknowledgments
We thank all the patients and families who participated in this trial, and we are indebted to the physicians and all of the clinical study teams at the participating institutions. We also thank Ms. Sanae Sakamoto for her excellent secretarial assistance at the data center of Clinical Research Support Center Kyushu.
Conflict of interest
The authors declare that they have no conflict of interest. All co-authors have seen and agree with the contents of the manuscript, and there are no financial interests to report. We certify that the submission presents original work and is not under review by any other publications.
Author information
Authors and Affiliations
Consortia
Corresponding author
About this article
Cite this article
Miwa, K., Oki, E., Emi, Y. et al. Phase II trial of an alternating regimen consisting of first-line mFOLFOX6 plus bevacizumab and FOLFIRI plus bevacizumab for patients with metastatic colorectal cancer: FIREFOX plus bevacizumab trial (KSCC0801). Int J Clin Oncol 21, 110–117 (2016). https://doi.org/10.1007/s10147-015-0850-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-015-0850-3