Abstract
Background
The randomized, double-blind, placebo-controlled GRID trial tested the oral multikinase inhibitor regorafenib in 199 patients with advanced gastrointestinal stromal tumors (GIST) following failure of at least imatinib and sunitinib, and showed a significant improvement in progression-free survival (PFS) versus placebo [hazard ratio (HR) 0.27; 95 % confidence interval (CI) 0.19–0.39; p < 0.0001].
Methods
A subgroup analysis of Japanese patients in the GRID study was performed to compare the efficacy and safety of oral regorafenib 160 mg once daily with matching placebo, in combination with best supportive care. The primary study endpoint was progression-free survival (PFS); safety was evaluated through the incidence of adverse events (AEs).
Results
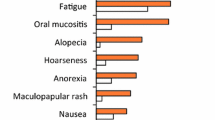
Seventeen Japanese patients were randomized to regorafenib (n = 12) or placebo (n = 5). Patient demographics were consistent with those of the overall study population. PFS was significantly longer with regorafenib than placebo (HR 0.08; 95 % CI 0.02–0.45; p = 0.000164). Centrally assessed disease control rates were 58 % and 20 % in the regorafenib and placebo groups, respectively (p = 0.080796). Treatment-related adverse events (AEs) were reported in all regorafenib-treated patients and 60 % of placebo recipients; the most frequent AE was hand–foot skin reaction (HFSR) (92 % versus 20 %, respectively).
Conclusion
Regorafenib showed efficacy and a manageable safety profile in Japanese patients with advanced GIST, consistent with the overall GRID study population. AEs, such as HFSR and maculopapular rash, were observed more frequently in Japanese patients. Although dose modification was frequently reported, only one patient with hepatic failure discontinued regorafenib because of AEs.




Similar content being viewed by others
References
Blay JY, Le Cesne A, Cassier PA et al (2012) Gastrointestinal stromal tumors (GIST): a rare entity, a tumor model for personalized therapy, and yet ten different molecular subtypes. Discov Med 13:357–367
Nilsson B, Bumming P, Meis-Kindblom JM et al (2005) Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era: a population-based study in western Sweden. Cancer (Phila) 103:821–829
Tryggvason G, Gislason HG, Magnusson MK et al (2005) Gastrointestinal stromal tumors in Iceland, 1990–2003: the Icelandic GIST study, a population-based incidence and pathologic risk stratification study. Int J Cancer 117:289–293
Nishida T, Takahashi T, Miyazaki Y (2009) Gastrointestinal stromal tumor: a bridge between bench and bedside. Gastric Cancer 12:175–188
Corless CL, Barnett CM, Heinrich MC (2011) Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer 11:865–878
Demetri GD, Benjamin RS, Blanke CD et al (2007) NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST): update of the NCCN clinical practice guidelines. J Natl Comp Canc Netw 5:S1–S29
Blay JY (2011) A decade of tyrosine kinase inhibitor therapy: historical and current perspectives on targeted therapy for GIST. Cancer Treat Rev 37:373–384
Demetri GD, von Mehren M, Antonescu CR et al (2010) NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Comp Canc Netw 8(suppl 2):S1–S41
Wilhelm SM, Dumas J, Adnane L et al (2011) Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 129:245–255
Demetri GD, Reichardt P, Kang Y-K et al (2013) Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381:295–302
US Food and Drug Administration (2013) FDA news release: FDA approves Stivarga for advanced gastrointestinal stromal tumors. US FDA. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm340958.htm. Accessed Aug 2014
Bayer HealthCare (2013) Bayer’s Stivarga® approved for the treatment of patients with gastrointestinal stromal tumors in Japan. http://press.healthcare.bayer.com/en/press/auth/news-details-page.php/15176/2013-0432. Accessed Aug 2014
Kim HS, Hong MH, Kim K et al (2011) Sunitinib for Asian patients with advanced renal cell carcinoma: a comparable efficacy with different toxicity profiles. Oncology 80:395–405
Uemura H, Shinohara N, Yuasa T et al (2010) A phase II study of sunitinib in Japanese patients with metastatic renal cell carcinoma: insights into the treatment, efficacy and safety. Jpn J Clin Oncol 40:194–202
Ueda T, Uemura H, Tomita Y et al (2013) Efficacy and safety of axitinib versus sorafenib in metastatic renal cell carcinoma: subgroup analysis of Japanese patients from the global randomized phase 3 AXIS trial. Jpn J Clin Oncol 43:616–628
Agaram NP, Besmer P, Wong GC et al (2007) Pathologic and molecular heterogeneity in imatinib-stable or imatinib-responsive gastrointestinal stromal tumors. Clin Cancer Res 13:170–181
Demetri GD, Jeffers M, Reichardt P et al (2013) Detection of oncogenic kinase mutations in circulating plasma DNA and correlation with clinical benefit in the phase III GRID study of regorafenib vs. placebo in TKI-refractory metastatic GIST. Cancer Res 73:LB–295 (abstract)
Cheng AL, Kang YK, Chen Z et al (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10:25–34
Yoshino T, Komatsu Y, Yamada Y et al (2014) Randomized phase III trial of regorafenib in metastatic colorectal cancer: analysis of the CORRECT Japanese and non-Japanese subpopulations. Invest New Drugs (Epub ahead of print, 12 Sep 2014)
Li J, Qin S, Yau T et al (2014) CONCUR: a randomized, double-blind, placebo-controlled phase 3 study of regorafenib monotherapy in Asian patients with previously treated metastatic colorectal cancer (mCRC). Ann Oncol 25(Suppl 2):ii114–ii115
Acknowledgments
We thank the participating patients and staff at each of the study centers. Editorial assistance in the preparation of this manuscript was provided by Succinct Medical Communications, with financial support from Bayer Health Care Pharmaceuticals; the authors retained editorial control over the content.
Conflict of interest
Yoshito Komatsu has received honoraria and research funding from Bayer. Yasuhide Yamada has received honoraria from Taiho, Chugai, and Pfizer and research funding from Novartis, Astrazeneca, Otsuka, MerckSerono, Chugai, and Daiichi-Sankyo. Iris Kuss owns stocks in Bayer. George Demetri has acted as a consultant to Kolltan Pharmaceuticals, Blueprint Medicines, G1 Therapeutics, Caris, Champions Oncology, and Bessor Pharmaceuticals (uncompensated), Pfizer, EMD-Serono, Sanofi Oncology, Janssen Oncology, Glaxo-Smith-Kline, Ariad, Astra-Zeneca, WIRB Copernicus Group, and Bayer. He has received research support from Bayer, Novartis, Pfizer, EMD-Serono, Sanofi Oncology, Janssen Oncology and Glaxo-Smith-Kline. He has equity in Kolltan Pharmaceuticals, Blueprint Medicines, G1 Therapeutics, Caris, Champions Oncology, and Bessor Pharmaceuticals. He has been part of scientific advisory boards for Kolltan Pharmaceuticals, Blueprint Medicines, G1 Therapeutics, Caris, and Champions Oncology and has provided noncompensated expert regulatory testimony on behalf of Bayer and Glaxo-Smith-Kline. Toshirou Nishida has received honoraria from Novartis, Pfizer, and Bayer and a research grant from Novartis. Toshihiko Doi, Akira Sawaki, and Tatsuo Kanda have no relevant conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Komatsu, Y., Doi, T., Sawaki, A. et al. Regorafenib for advanced gastrointestinal stromal tumors following imatinib and sunitinib treatment: a subgroup analysis evaluating Japanese patients in the phase III GRID trial. Int J Clin Oncol 20, 905–912 (2015). https://doi.org/10.1007/s10147-015-0790-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-015-0790-y