Abstract
Background
[18F]fluoro-2-deoxyglucose-positron emission tomography (FDG-PET) is widely used to evaluate tumor metabolic activity. The aim of this study was to evaluate the usefulness of FDG-PET in assessing the histopathological response to preoperative concurrent chemoradiotherapy (CRT) in patients with oral squamous cell carcinoma (OSCC).
Methods
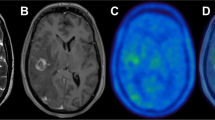
Forty-five patients with resectable advanced OSCC who had received preoperative CRT followed by tumor ablative surgery between January 2004 and December 2011 were included in the study. All patients underwent FDG-PET before and after preoperative CRT. The maximum standardized uptake value (SUVmax) before (pre-SUV) and after preoperative CRT (post-SUV) and the SUVmax reduction rate (ΔSUV %) were used to evaluate the response to preoperative CRT. Correlations among SUVmax, histopathological response, and expression of cancer antigen Ki-67 and hypoxia-inducible factor-1α (HIF-1α) were analyzed.
Results
Preoperative CRT significantly reduced intratumoral FDG uptake (P < 0.001). The pre-SUV and post-SUV were significantly lower in patients with a pathological complete response (pCR) than in those with a non-pCR (pre-SUV P = 0.037; post-SUV P = 0.001). ΔSUV % was higher in patients with pCR than in those with non-pCR (P = 0.029). The pre-SUV was significantly correlated with Ki-67 and HIF-1α expression in pretreatment biopsy specimens (Ki-67 P = 0.046, R = 0.292; HIF-1α P = 0.007, R = 0.385). The expression of both Ki-67 and HIF-1α was significantly lower in patients with pCR than in those with non-pCR (Ki-67 P < 0.001; HIF-1α P < 0.001).
Conclusions
Low pre-SUV and post-SUV and high ΔSUV % may predict a good histopathological response to preoperative CRT. Ki-67 and HIF-1α expression in pretreatment biopsy specimens were predictors of histopathological response to preoperative CRT.





Similar content being viewed by others
References
Japan Society for Head and Neck Cancer Cancer Registry Committee (2006) Report of head and neck cancer registry of Japan clinical statistics of registered patients, 2002. Jpn J Head Neck Cancer 32:1–98
Izumo T, Kirita T, Ariji E et al (2012) General rules for clinical and pathological studies on oral cancer: a synopsis. Jpn J Clin Oncol 42:1099–1109
Pignon JP, Bourhis J, Domenge C et al (2000) Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of chemotherapy on head and neck cancer. Lancet 355:949–955
Kirita T, Ohgi K, Shimooka H et al (1999) Preoperative concurrent chemoradiotherapy plus radical surgery for advanced squamous cell carcinoma of the oral cavity: an analysis of long-term results. Oral Oncol 35:597–606
Yamanaka Y, Tamaki S, Shimomura H et al (2011) A case of advanced upper gingival carcinoma responding completely to concurrent chemoradiotherapy with S-1. Gan To Kagaku Ryoho 38:89–92 (in Japanese)
Klug C, Berzaczy D, Voracek M et al (2008) Preoperative chemoradiotherapy in the management of oral cancer: a review. J Craniomaxillofac Surg 36:75–88
Eckardt A, Sinikovic B, Hofele C et al (2007) Preoperative paclitaxel/carboplatin radiochemotherapy for stage III/IV resectable oral and oropharyngeal cancer: seven-year follow-up of a phase II trial. Oncology 73:198–203
Kirita T, Yamanaka Y, Imai Y et al (2012) Preoperative concurrent chemoradiotherapy for stages II-IV oral squamous cell carcinoma: a retrospective analysis and the future possibility of this treatment strategy. Int J Oral Maxillofac Surg 41:421–428
Kitagawa Y, Sano K, Nishizawa S et al (2003) FDG-PET for prediction of tumour aggressiveness and response to intra-arterial chemotherapy and radiotherapy in head and neck cancer. Eur J Nucl Med Mol Imaging 30:63–71
Lee SW, Nam SY, Im KC et al (2008) Prediction of prognosis using standardized uptake value of 2-[(18)F] fluoro-2-deoxy-d-glucose positron emission tomography for nasopharyngeal carcinomas. Radiother Oncol 87:211–216
Kim MK, Ryu JS, Kim SB et al (2007) Value of complete metabolic response by (18)F-fluorodeoxyglucose-positron emission tomography in oesophageal cancer for prediction of pathologic response and survival after preoperative chemoradiotherapy. Eur J Cancer 43:1385–1391
Higashi K, Ueda Y, Arisaka Y et al (2002) 18F-FDG uptake as a biologic prognostic factor for recurrence in patients with surgically resected non-small cell lung cancer. J Nucl Med 43:39–45
Shimosato Y, Obishi S, Baba K (1971) Histological evaluation of effects of radiotherapy and chemotherapy for carcinomas. Jpn J Clin Oncol 1:19–35
Sasahira T, Ueda N, Yamamoto K et al (2013) Trks are novel oncogenes involved in the induction of neovascularization, tumor progression, and nodal metastasis in oral squamous cell carcinoma. Clin Exp Metastasis 30:165–176
Ishihara R, Yamamoto S, Iishi H et al (2012) Predicting the effects of chemoradiotherapy for squamous cell carcinoma of the esophagus by induction chemotherapy response assessed by positron emission tomography: toward PET-response-guided selection of chemoradiotherapy or esophagectomy. Int J Clin Oncol 17:225–232
Kresnik E, Mikosch P, Gallowitsch HJ et al (2001) Evaluation of head and neck cancer with 18F-FDG PET: a comparison with conventional methods. Eur J Nucl Med 28:816–821
Kim SY, Kim JS, Doo H et al (2011) Combined [18F] fluorodeoxyglucose positron emission tomography and computed tomography for detecting contralateral neck metastases in patients with head and neck squamous cell carcinoma. Oral Oncol 47:376–380
Vaupel P, Thews O, Hoeckel M (2001) Treatment resistance of solid tumors: role of hypoxia and anemia. Med Oncol 18:243–259
Nguyen XC, Lee WW, Chung JH et al (2007) FDG uptake, glucose transporter type 1, and Ki-67 expressions in non-small-cell lung cancer: correlations and prognostic values. Eur J Radiol 62:214–219
Jalava P, Kuopio T, Juntti-Patinen L et al (2006) Ki67 immunohistochemistry: a valuable marker in prognostication but with a risk of misclassification: proliferation subgroups formed based on Ki67 immunoreactivity and standardized mitotic index. Histopathology 48:674–682
Viale G, Giobbie-Hurder A, Regan MM et al (2008) Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1-98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol 26:5569–5575
Pugsley JM, Schmidt RA, Vesselle H (2002) The Ki-67 index and survival in non-small cell lung cancer: a review and relevance to positron emission tomography. Cancer J 8:222–233
Harada H, Kizaka-Kondoh S, Li G et al (2007) Significance of HIF-1-active cells in angiogenesis and radioresistance. Oncogene 26:7508–7516
Song X, Liu X, Chi W et al (2006) Hypoxia-induced resistance to cisplatin and doxorubicin in non-small cell lung cancer is inhibited by silencing of HIF-1alpha gene. Cancer Chemother Pharmacol 58:776–784
García Vicente AM, Castrejón ÁS, Relea Calatayud F et al (2012) 18F-FDG retention index and biologic prognostic parameters in breast cancer. Clin Nucl Med 37:460–466
Yamada T, Uchida M, Kwang-Lee K et al (2012) Correlation of metabolism/hypoxia markers and fluorodeoxyglucose uptake in oral squamous cell carcinomas. Oral Surg Oral Med Oral Pathol Oral Radiol 113:464–471
Oshida M, Uno K, Suzuki M et al (1998) Predicting the prognoses of breast carcinoma patients with positron emission tomography using 2-deoxy-2-fluoro[18F]-d-glucose. Cancer 82:2227–2234
Vansteenkiste JF, Stroobants SG, Dupont PJ et al (1999) Prognostic importance of the standardized uptake value on (18)F-fluoro-2-deoxy-glucose-positron emission tomography scan in non-small-cell lung cancer: an analysis of 125 cases. Leuven Lung Cancer Group. J Clin Oncol 17:3201–3206
Brun E, Kjellén E, Tennvall J et al (2002) FDG PET studies during treatment: prediction of therapy outcome in head and neck squamous cell carcinoma. Head Neck 24:127–135
Vallbohmer D, Holscher AH, Dietlein M et al (2009) [18F]-Fluorodeoxyglucose-positron emission tomography for the assessment of histopathologic response and prognosis after completion of neoadjuvant chemoradiation in esophageal cancer. Ann Surg 250:888–894
Miyawaki A, Ikeda R, Hijioka H et al (2010) SUVmax of FDG-PET correlates with the effects of neoadjuvant chemoradiotherapy for oral squamous cell carcinoma. Oncol Rep 23:1205–1212
Jingu K, Kaneta T, Nemoto K et al (2010) (18)F-fluorodeoxyglucose positron emission tomography immediately after chemoradiotherapy predicts prognosis in patients with locoregional postoperative recurrent esophageal cancer. Int J Clin Oncol 15:184–190
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Shimomura, H., Sasahira, T., Yamanaka, Y. et al. [18F]fluoro-2-deoxyglucose-positron emission tomography for the assessment of histopathological response after preoperative chemoradiotherapy in advanced oral squamous cell carcinoma. Int J Clin Oncol 20, 308–316 (2015). https://doi.org/10.1007/s10147-014-0711-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-014-0711-5