Abstract
Background
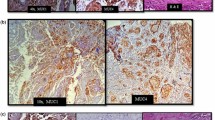
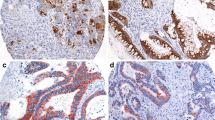
Both MUC1 and MUC4 are high molecular weight glycoproteins and are independent indicators of worse prognosis in many human epithelial cancers including oral squamous cell carcinoma (OSCC). However, there has been no investigation of the clinical importance of the co-expression of MUC1 and MUC4 in OSCC. The aim of this study was to evaluate the co-expression profile of MUC1/MUC4 and analyze the prognostic significance in OSCC.
Methods
We examined the expression profile of MUC1 and MUC4 in OSCC tissues from 206 patients using immunohistochemistry. The co-expression profile of MUC1/MUC4 and its prognostic significance in OSCC was statistically analyzed.
Results
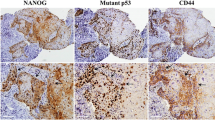
MUC1 and MUC4 overexpression were strongly correlated with each other (p < 0.0001) and a combination of both MUC1 and MUC4 expression was a powerful indicator for tumor aggressiveness such as tumor size (p = 0.014), lymph node metastasis (0.0001), tumor stage (p = 0.006), diffuse invasion (p = 0.028), and vascular invasion (p = 0.014). The MUC1/MUC4 double-positive patients showed the poorest overall and disease-free survival. Multivariate analysis revealed that MUC1/MUC4 double-positivity was the strong independent prognostic factor for overall and disease-free survival (p = 0.007 and (p = 0.0019), in addition to regional recurrence (p = 0.0025).
Conclusions
Taken together, these observations indicate that the use of a combination of MUC1/MUC4 can predict outcomes for patients with OSCC. This combination is also a useful marker for predicting regional recurrence. MUC1 and MUC4 may be attractive targets for the selection of treatment methods in OSCC.




Similar content being viewed by others
References
Jemal A, Bray F, Center MM et al (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Warnakulasuriya S (2009) Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 45:309–316
Cheng A, Schmidt BL (2008) Management of the N0 neck in oral squamous cell carcinoma. Oral Maxillofac Surg Clin N Am 20:477–497
Kowalski LP, Bagietto R, Lara JR et al (2000) Prognostic significance of the distribution of neck node metastasis from oral carcinoma. Head Neck 22:207–214
Capote A, Escorial V, Munoz-Guerra MF et al (2007) Elective neck dissection in early-stage oral squamous cell carcinoma—does it influence recurrence and survival? Head Neck 29:3–11
Mahfouz ME, Rodrigo JP, Takes RP et al (2010) Current potential and limitations of molecular diagnostic methods in head and neck cancer. Eur Arch Otorhinolaryngol 267:851–860
Hollingsworth MA, Swanson BJ (2004) Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer 4:45–60
Moniaux N, Escande F, Porchet N et al (2001) Structural organization and classification of the human mucin genes. Front Biosci 6:1192–1206
Kufe DW (2009) Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer 9:874–885
Shibahara H, Tamada S, Higashi M et al (2004) MUC4 is a novel prognostic factor of intrahepatic cholangiocarcinoma-mass forming type. Hepatology 39:220–229
Higashi M, Yonezawa S, Ho JJ et al (1999) Expression of MUC1 and MUC2 mucin antigens in intrahepatic bile duct tumors: its relationship with a new morphological classification of cholangiocarcinoma. Hepatology 30:1347–1355
Nakamura A, Horinouchi M, Goto M et al (2002) New classification of pancreatic intraductal papillary-mucinous tumour by mucin expression: its relationship with potential for malignancy. J Pathol 197:201–210
Kitazono I, Higashi M, Kitamoto S et al (2013) Expression of MUC4 mucin is observed mainly in the intestinal type of intraductal papillary mucinous neoplasm of the pancreas. Pancreas 42:1120–1128
Osako M, Yonezawa S, Siddiki B et al (1993) Immunohistochemical study of mucin carbohydrates and core proteins in human pancreatic tumors. Cancer 71:2191–2199
Sagara M, Yonezawa S, Nagata K et al (1999) Expression of mucin 1 (MUC1) in esophageal squamous-cell carcinoma: its relationship with prognosis. Int J Cancer 84:251–257
Takao S, Uchikura K, Yonezawa S et al (1999) Mucin core protein expression in extrahepatic bile duct carcinoma is associated with metastases to the liver and poor prognosis. Cancer 86:1966–1975
Saitou M, Goto M, Horinouchi M et al (2005) MUC4 expression is a novel prognostic factor in patients with invasive ductal carcinoma of the pancreas. J Clin Pathol 58:845–852
Shibahara H, Tamada S, Goto M et al (2004) Pathologic features of mucin-producing bile duct tumors: two histopathologic categories as counterparts of pancreatic intraductal papillary-mucinous neoplasms. Am J Surg Pathol 28:327–338
Tamada S, Goto M, Nomoto M et al (2002) Expression of MUC1 and MUC2 mucins in extrahepatic bile duct carcinomas: its relationship with tumor progression and prognosis. Pathol Int 52:713–723
Tamada S, Shibahara H, Higashi M et al (2006) MUC4 is a novel prognostic factor of extrahepatic bile duct carcinoma. Clin Cancer Res 12:4257–4264
Tamura Y, Higashi M, Kitamoto S et al (2012) MUC4 and MUC1 expression in adenocarcinoma of the stomach correlates with vessel invasion and lymph node metastasis: an immunohistochemical study of early gastric cancer. PLoS One 7:e49251
Tsutsumida H, Goto M, Kitajima S et al (2007) MUC4 expression correlates with poor prognosis in small-sized lung adenocarcinoma. Lung Cancer 55:195–203
Yonezawa S, Goto M, Yamada N et al (2008) Expression profiles of MUC1, MUC2, and MUC4 mucins in human neoplasms and their relationship with biological behavior. Proteomics 8:3329–3341
Yonezawa S, Higashi M, Yamada N et al (2010) Significance of mucin expression in pancreatobiliary neoplasms. J Hepatobiliary Pancreat Sci 17:108–124
Yonezawa S, Higashi M, Yamada N et al (2008) Precursor lesions of pancreatic cancer. Gut Liver 2:137–154
Yonezawa S, Taira M, Osako M et al (1998) MUC-1 mucin expression in invasive areas of intraductal papillary mucinous tumors of the pancreas. Pathol Int 48:319–322
Yonezawa S, Higashi M, Yamada N et al (2011) Mucins in human neoplasms: clinical pathology, gene expression and diagnostic application. Pathol Int 61:697–716
Yonezawa S, Sato E (1997) Expression of mucin antigens in human cancers and its relationship with malignancy potential. Pathol Int 47:813–830
Yonezawa S, Nakamura A, Horinouchi M et al (2002) The expression of several types of mucin is related to the biological behavior of pancreatic neoplasms. J Hepatobiliary Pancreat Surg 9:328–341
Hamada T, Nomura M, Kamikawa Y et al (2012) DF3 epitope expression on MUC1 mucin is associated with tumor aggressiveness, subsequent lymph node metastasis, and poor prognosis in patients with oral squamous cell carcinoma. Cancer 118:5251–5264
Hamada T, Wakamatsu T, Miyahara M et al (2012) MUC4: a novel prognostic factor of oral squamous cell carcinoma. Int J Cancer 130:1768–1776
Lee HK, Cho MS, Kim TH (2012) Prognostic significance of muc4 expression in gallbladder carcinoma. World J Surg Oncol 10:224
Sobin LGM, Wittekind Ce (2009) TNM Classification of malignant tumors. In: Hoboken NJ (ed), 7th ed John Wiley & Sons, Inc.
Yamamoto E, Kohama G, Sunakawa H et al (1983) Mode of invasion, bleomycin sensitivity, and clinical course in squamous cell carcinoma of the oral cavity. Cancer 51:2175–2180
Hayes DF, Sekine H, Ohno T et al (1985) Use of a murine monoclonal antibody for detection of circulating plasma DF3 antigen levels in breast cancer patients. J Clin Invest 75:1671–1678
Nagy P, Friedländer E, Tanner M et al (2005) Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a Herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res 65:473–482
Singh AP, Chaturvedi P, Batra SK (2007) Emerging roles of MUC4 in cancer: a novel target for diagnosis and therapy. Cancer Res 67:433–436
Yamada N, Nishida Y, Tsutsumida H et al (2008) MUC1 expression is regulated by DNA methylation and histone H3 lysine 9 modification in cancer cells. Cancer Res 68:2708–2716
Yamada N, Nishida Y, Tsutsumida H et al (2009) Promoter CpG methylation in cancer cells contributes to the regulation of MUC4. Br J Cancer 100:344–351
Vincent A, Ducourouble MP, Van Seuningen I (2008) Epigenetic regulation of the human mucin gene MUC4 in epithelial cancer cell lines involves both DNA methylation and histone modifications mediated by DNA methyltransferases and histone deacetylases. FASEB J 22:3035–3045
Zhang L, Vlad A, Milcarek C et al (2013) Human mucin MUC1 RNA undergoes different types of alternative splicing resulting in multiple isoforms. Cancer Immunol Immunother 62:423–435
Obermair A, Schmid BC, Packer LM et al (2002) Expression of MUC1 splice variants in benign and malignant ovarian tumours. Int J Cancer 100:166–171
Schmid BC, Rudas M, Fabjani G et al (2003) Evaluation of MUC1 splice variants as prognostic markers in patients with ductal carcinoma in situ of the breast. Oncol Rep 10:1981–1985
Choudhury A, Moniaux N, Winpenny JP et al (2000) Human MUC4 mucin cDNA and its variants in pancreatic carcinoma. J Biochem 128:233–243
Moniaux N, Escande F, Batra SK et al (2000) Alternative splicing generates a family of putative secreted and membrane-associated MUC4 mucins. Eur J Biochem 267:4536–4544
Choudhury A, Moniaux N, Ringel J et al (2001) Alternate splicing at the 30-end of the human pancreatic tumor-associated mucin MUC4 cDNA. Teratog Carcinog Mutagen 21:83–96
Acknowledgments
The authors thank Mr. Yoshiharu Atsuji and Ms. Yukari Nishimura for their excellent technical assistance. This study was supported in part by Grants-in-Aid for Scientific Research (B) 23390085 to S. Yonezawa; Scientific Research (C) 24590447 to M. Higashi; Scientific Research (B) 23390466 to K. Sugihara and Young Scientists (B) 24792239 to T. Hamada from the Ministry of Education, Science, Sports, Culture and Technology, Japan.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Y. Kamikawa and Y. Kanmura contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Kamikawa, Y., Kanmura, Y., Hamada, T. et al. Combination of MUC1 and MUC4 expression predicts clinical outcome in patients with oral squamous cell carcinoma. Int J Clin Oncol 20, 298–307 (2015). https://doi.org/10.1007/s10147-014-0710-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-014-0710-6