Abstract
Objectives
This study was conducted to evaluate the efficacy and safety of S-1 in patients with advanced non-small-cell lung cancer (NSCLC), receiving two or more prior chemotherapy regimens.
Methods
S-1 was administered orally for 14 consecutive days, followed by a 7-day rest period. This treatment course was repeated until disease progression or intolerable toxicity occurred.
Results
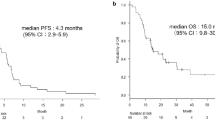
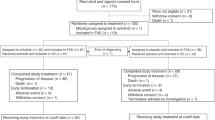
From 2010 to 2012, 45 patients were enrolled in this study. Of the 45 patients, 4 patients [8.9 %, 95 % confidence interval (CI) 0.6–17.2 %] exhibited a partial response and 24 patients (53.3 %) exhibited stable disease. The disease control rate was 62.2 % (95 % CI 48.1–76.4 %). Median progression-free survival was 71 days, and median survival time was 205 days. Four patients had grade 3 hematological toxicities, but toxicities of grade 4 were not observed in this study.
Conclusion
Although S-1 monotherapy as third-line treatment or beyond was well tolerated, the response rate for this regimen did not demonstrate sufficient activity for patients with advanced NSCLC.

Similar content being viewed by others
References
Jemal A, Siegal R, Ward E et al (2009) Cancer statistics, 2009. CA Cancer J Clin 59:225–249
Shiroyama T, Kijima T, Komuta K et al (2012) Phase II tailored S-1 regimen study of first-line chemotherapy in elderly patients with advanced and recurrent non-small cell lung cancer. Cancer Chemother Pharmacol 70:783–789
Morgensztern D, Ng SH, Gao F et al (2010) Trends in stage distribution for patients with non-small cell lung cancer: a National Cancer Database survey. J Thorac Oncol 5:29–33
Schiller JH, Harrington D, Belani CP, Eastern Cooperative Oncology Group et al (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346:92–98
Ohe Y, Ohashi Y, Kubota K et al (2007) Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: Four-Arm Cooperative Study in Japan. Ann Oncol 18:317–323
Shepherd FA, Dancey J, Ramlau R et al (2000) Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 18:2095–2103
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, National Cancer Institute of Canada Clinical Trials Group et al (2005) Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353:123–132
Hanna N, Shepherd FA, Fossella FV et al (2004) Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 22:1589–1597
Matsubara N, Maemondo M, Inoue A et al (2013) A phase II study of irinotecan as a third- or fourth-line treatment for advanced non-small cell lung cancer: NJLCG0703. Respir Investig 51:28–34
Harada T, Oizumi S, Ito K, Hokkaido Lung Cancer Clinical Study Group et al (2013) A phase II study of amrubicin as a third-line or fourth-line chemotherapy for patients with non-small cell lung cancer: Hokkaido lung cancer clinical study group trial (HOT) 0901. Oncologist 18:439–445
Shirasaka T, Nakano K, Takechi T et al (1996) Antitumor activity of 1 M tegafur-0.4 M 5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against human colon carcinoma orthotopically implanted into nude rats. Cancer Res 56:2602–2606
Takechi T, Nakano K, Uchida J et al (1997) Antitumor activity and low intestinal toxicity of S-1, a new formulation of oral tegafur, in experimental tumor models in rats. Cancer Chemother Pharmacol 39:205–211
Govindan R, Morgensztern D, Kommor MD et al (2011) Phase II trial of S-1 as second-line therapy in patients with advanced non-small cell lung cancer. J Thorac Oncol 6:790–795
Hashizume T, Nakada Y (2009) S-1 monotherapy in patients with pretreated advanced non-small cell lung cancer. Gan To Kagaku Ryoho 36:963–967
Shiroyama T, Komuta K, Imamura F et al (2011) Phase II study of S-1 monotherapy in platinum-refractory, advanced non-small cell lung cancer. Lung Cancer 74:85–88
Totani Y, Saito Y, Hayashi M et al (2009) A phase II study of S-1 monotherapy as second-line treatment for advanced non-small cell lung cancer. Cancer Chemother Pharmacol 64:1181–1185
Wada M, Yamamoto M, Ryuge S et al (2012) Phase II study of S-1 monotherapy in patients with previously treated, advanced non-small-cell lung cancer. Cancer Chemother Pharmacol 69:1005–1011
Takakuwa O, Oguri T, Maeno K et al (2010) Efficacy of S-1 monotherapy for non-small cell lung cancer after the failure of two or more prior chemotherapy regimens. Oncol Lett 1:147–150
Ono A, Naito T, Murakami H et al (2010) Evaluation of S-1 as third- or further-line chemotherapy in advanced non-small-cell lung cancer. Int J Clin Oncol 15:161–165
Massarelli E, Andre F, Liu DD et al (2003) A retrospective analysis of the outcome of patients who have received two prior chemotherapy regimens including platinum and docetaxel for recurrent non-small-cell lung cancer. Lung Cancer 39:55–61
Yoshioka H, Okamoto I, Morita S et al (2013) Efficacy and safety analysis according to histology for S-1 in combination with carboplatin as first-line chemotherapy in patients with advanced non-small-cell lung cancer: updated results of the West Japan Oncology Group LETS study. Ann Oncol 24:1326–1331
Kawahara M, Furuse K, Segawa Y, S-1 Cooperative Study Group (Lung Cancer Working Group) et al (2001) Phase II study of S-1, a novel oral fluorouracil, in advanced non-small-cell lung cancer. Br J Cancer 85:939–943
Furuse K, Kawahara M, Hasegawa K, S-1 Cooperative Study Group (Lung Cancer Working Group) et al (2001) Early phase II study of S-1, a new oral fluoropyrimidine, for advanced non-small-cell lung cancer. Int J Clin Oncol 6:236–241
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Miyoshi, S., Ito, R., Katayama, H. et al. Phase II trial of S-1 as third-line or further chemotherapy in patients with advanced non-small-cell lung cancer. Int J Clin Oncol 19, 1005–1010 (2014). https://doi.org/10.1007/s10147-014-0663-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-014-0663-9