Abstract
Background
Cancer subtype has recently become an increasingly important consideration when deciding the treatment strategy for breast cancer. For the estrogen receptor positive (ER+) subtype, the efficacy of adjuvant endocrine therapy is definitive, but that of adjuvant chemotherapy is controversial.
Methods
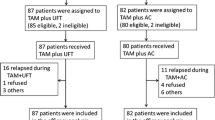
In order to evaluate the effect of adding doxorubicin (A) and cyclophosphamide (C) to tamoxifen (TAM) (ACT) on the overall survival (OS) of node-positive postmenopausal breast cancer (PMBC) patients, we conducted a randomized trial. Eligibility criteria included pathologically node-positive (n = 1–9) PMBC, stage I–IIIA disease. Patients were randomized to receive either TAM (20 mg daily) for 2 years or A (40 mg/m2) and C (500 mg/m2) plus TAM (ACT) as adjuvant therapy following surgery.
Results
One hundred twenty-nine patients were recruited (TAM 64, ACT 65) between October 1994 and July 1999. The hazard ratios for OS and relapse-free survival (RFS) were 0.58 (95 % CI 0.24–1.39; log-rank p = 0.22) and 0.45 (95 %CI 0.24–0.86; log-rank p = 0.013), respectively, in favor of ACT. The 5-year OS and RFS were 76.9 % (ER+ 87.1 %, ER− 53.3 %) and 54.9 % (ER+ 59.3 %, ER− 42.9 %) for TAM and 85.0 % (ER+ 90.0 %, ER− 77.1 %) and 76.7 % (ER+ 76.9 %, ER− 76.0 %) for ACT. A higher proportion of the patients receiving ACT than those receiving TAM experienced grade 3 decreased white blood cell count and grade 2–3 nausea.
Conclusion
The efficacy of adding AC to TAM was not high for ER+, node-positive PMBC. However, adjuvant ACT therapy was considered to be effective for ER−, node-positive PMBC.



Similar content being viewed by others
References
Early Breast Cancer Trialists’ Collaborative Group (1990) Treatment of early breast cancer: worldwide evidence in 1985–1990. A systematic overview of all available randomized trials in early breast cancer of adjuvant endocrine and cytotoxic therapy-treatment of early breast cancer, vol 1. Oxford University Press, Oxford, pp 21–49
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472):1687–1717
Consensus conference (1985) Adjuvant chemotherapy for breast cancer. JAMA 254:3461–3463
Glick JH, Gelber RD, Goldhirsch A et al (1992) Meeting highlights: adjuvant therapy for primary breast cancer. J Natl Cancer Inst 84:1479–1485
Fisher B, Redmond C, Legault-Poisson S et al (1990) Postoperative chemotherapy and tamoxifen compared with tamoxifen alone in the treatment of positive node breast cancer patients aged 50 years and older with tumors responsive to tamoxifen: results from the national surgical adjuvant breast and bowel project B-16. J Clin Oncol 8:1005–1018
Tobinai K, Kohno A, Shimada Y et al (1993) Toxicity grading criteria of the Japan Clinical Oncology Group. The Clinical Trial Review Committee of the Japan Clinical Oncology Group. Jpn J Clin Oncol 23(4):250–257
Gelber RD, Goldhirsch A (1986) A new endpoint for the assessment of adjuvant therapy in postmenopausal women with operable breast cancer. J Clin Oncol 4:1772–1779
Boccardo F, Rubagotti A, Amoroso D et al (1992) Chemotherapy versus tamoxifen versus chemotherapy plus tamoxifen in node positive, oestrogen receptor positive breast cancer patients. An update at 7 years of the 1st GROCTA Trial. Eur J Cancer 28:673–680
Pearson OH, Hubay CA, Gordon NH et al (1989) Endocrine versus endocrine plus five-drug chemotherapy in postmenopausal women with stage II estrogen receptor positive breast cancer. Cancer 64:1819–1823
Pritchard KI, Paterson AH, Paul NA et al (1996) Increased thromboembolic complications with concurrent tamoxifen and chemotherapy in a randomized trial of adjuvant therapy for women with breast cancer. National Cancer Institute of Canada Clinical Trials Group Breast Cancer Site Group. J Clin Oncol 14(10):2731–2737
Pagani O, Gelber S, Simoncini E et al (2009) Is adjuvant chemotherapy of benefit for postmenopausal women who receive endocrine treatment for highly endocrine-responsive, node-positive breast cancer? International Breast Cancer Study Group Trials VII and 12-93. Breast Cancer Res Treat 116(3):491–500
Fossati R, Confalonieri C, Torri V et al (1998) Cytotoxic and hormonal treatment for metastatic breast cancer: a systematic review of published randomized trials involving 31,510 women. J Clin Oncol 16(10):3439–3460
Fisher B, Anderson S, Tan-Chiu E et al (2001) Tamoxifen and chemotherapy for axillary node-negative, estrogen receptor negative breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-23. J Clin Oncol 19(4):931–942
Early Breast Cancer Trialists’ Collaborative Group (1998) Tamoxifen for early breast cancer: an overview of the randomized trials. Lancet 351(9114):1451–1467
Goldhirsch A, Wood WC, Coates AS et al (2011) Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 22(8):1736–1747
Paik S, Tang G, Shak S et al (2006) Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24(23):3726–3734
Albain KS, Barlow WE, Shak S et al (2010) Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 11(1):55–65
Hugh J, Hanson J, Cheang MC et al (2009) Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol 27:1168–1176
Penault-Llorca F, André F, Sagan C et al (2009) Ki-67 expression and docetaxel efficacy in patients with estrogen receptor-positive breast cancer. J Clin Oncol 27(17):2809–2815
Niikura N, Iwamoto T, Masuda S et al (2012) Immunohistochemical Ki67 labeling index has similar proliferation predictive power to various gene signatures in breast cancer. Cancer Sci 103(8):1508–1512
Dowsett M, Nielsen TO, A’Hern R et al (2011) Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer Working Group. JNCI. J Natl Cancer Inst 103:1656–1664
Acknowledgments
We thank Ms. Kyoko Minamoto and Kazumi Kubota for data management, Dr. Naoki Ishizuka and Mr. Junki Mizusawa for statistical analyses, and Dr. Kenichi Nakamura for the preparation of the manuscript. This study was supported by a National Cancer Center Research and Development Fund (23-A-16 and 23-A-17) and Grants-in-Aid for Cancer Research (5S-1, 8S-1, 11S-1, 11S-4, 14S-1, 14S-4, 17S-1, 17S-5, 20S-1 and 20S-6) from the Ministry of Health, Labour and Welfare, Japan.
Conflict of interest
Hiroji Iwata received honoraria for speaking events from Chugai Pharmaceutical Co., Ltd. Tadahiko Shien, Kenjiro Aogi, Takashi Fukutomi, Kenichi Inoue, Takayuki Kinoshita, Masato Takahashi, Akira Matsui, Taro Shibata, Haruhiko Fukuda had no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the JCOG Breast Cancer Study Group. The 22 institutions that belong to the JCOG Breast Cancer Study Group are listed in Appendix.
Appendix: Participating institutions (from north to south)
Appendix: Participating institutions (from north to south)
The 22 institutions that belonged to the JCOG Breast Cancer Study Group and participated in this trial are as follows: National Sapporo Hospital, International Medical Center of Japan, Tochigi Cancer Center, Metropolitan Komagome Hospital, National Cancer Center, National Cancer Center East, Tokai University Hospital, National Atami Hospital, Hamamatsu Medical Center, Aichi Cancer Center, Osaka National Hospital, Kinki University Hospital, National Shikoku Cancer Center, National Kure Medical Center, National Nagasaki Medical Center, Saitama Cancer Center, St Luke’s International Hospital, Hyogo Medical Center, Shizuoka Cancer Center, Niigata Cancer Center Hospital, Kawasaki Medical School Hospital, and Kitakyushu Municipal Medical Center.
About this article
Cite this article
Shien, T., Iwata, H., Aogi, K. et al. Tamoxifen versus tamoxifen plus doxorubicin and cyclophosphamide as adjuvant therapy for node-positive postmenopausal breast cancer: results of a Japan Clinical Oncology Group Study (JCOG9401). Int J Clin Oncol 19, 982–988 (2014). https://doi.org/10.1007/s10147-013-0657-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-013-0657-z