Abstract
Background
Recurrent glioblastoma after initial radiotherapy plus concomitant and adjuvant temozolomide is problematic. Here, patients with temozolomide-refractory high-grade gliomas were treated with bevacizumab (BV) and evaluated using apparent diffusion coefficient (ADC) for response.
Methods
Nine post-temozolomide recurrent or progressive high-grade glioma patients (seven with glioblastoma and two with anaplastic astrocytoma) were treated with BV monotherapy. Average age was 57 years (range, 22–78), median Karnofsky Performance Scale (KPS) was 70 (30–80) and median BV line number was 2 (2–5). Two had additional stereotactic radiotherapy within 6 months prior to BV. Magnetic resonance (MR) imaging after BV therapy was performed within 2 weeks with calculation of mean ADC (mADC) values of enhancing tumor contours.
Results
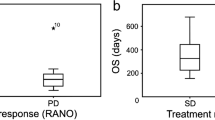
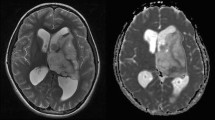
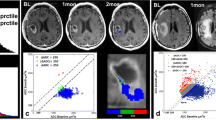
Post-BV treatment MR imaging showed decreased tumor volumes in eight of nine cases (88.9 %). Partial response was obtained in four cases (44.4 %), four cases had stable disease, and one had progressive disease. Of 15 evaluable enhancing lesions, 11 shrank and four did not. Pretreatment mADC values were above 1100 (10−6 mm2/s) in all responding tumors, while all non-responding lesions scored below 1100 (p = 0.001). mADC decreased after the first BV treatment in all lesions except one. KPS improved in four cases (44.4 %). Median progression-free survival and overall survival for those having all lesions with high mADC (>1100) were significantly longer than those with a low mADC (<1100) lesion (p = 0.018 and 0.046, respectively).
Conclusions
Bevacizumab monotherapy is effective in patients with temozolomide-refractory recurrent gliomas and tumor mean ADC value can be a useful marker for prediction of BV response and survival.




Similar content being viewed by others
References
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Norden AD, Drappatz J, Wen PY (2009) Antiangiogenic therapies for high-grade glioma. Nat Rev Neurol 5:610–620
Fischer I, Gagner JP, Law M et al (2005) Angiogenesis in gliomas: biology and molecular pathophysiology. Brain Pathol 15:297–310
Yuan F, Chen Y, Dellian M et al (1996) Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc Natl Acad Sci USA 93:14765–14770
Chamberlain MC (2010) Emerging clinical principles on the use of bevacizumab for the treatment of malignant gliomas. Cancer 116:3988–3999
Vredenburgh JJ, Desjardins A, Herndon JE 2nd et al (2007) Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 25:4722–4729
Friedman HS, Prados MD, Wen PY et al (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733–4740
Kreisl TN, Kim L, Moore K et al (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27:740–745
Sugahara T, Korogi Y, Kochi M et al (1999) Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging 9:53–60
Mardor Y, Roth Y, Ochershvilli A et al (2004) Pretreatment prediction of brain tumors’ response to radiation therapy using high b-value diffusion-weighted MRI. Neoplasia 6:136–142
Chenevert TL, Sundgren PC, Ross BD (2006) Diffusion imaging: insight to cell status and cytoarchitecture. Neuroimaging Clin N Am 16:619–632 viii-ix
Oh J, Henry RG, Pirzkall A et al (2004) Survival analysis in patients with glioblastoma multiforme: predictive value of choline-to-N-acetylaspartate index, apparent diffusion coefficient, and relative cerebral blood volume. J Magn Reson Imaging 19:546–554
Murakami R, Sugahara T, Nakamura H et al (2007) Malignant supratentorial astrocytoma treated with postoperative radiation therapy: prognostic value of pretreatment quantitative diffusion-weighted MR imaging. Radiology 243:493–499
Higano S, Yun X, Kumabe T et al (2006) Malignant astrocytic tumors: clinical importance of apparent diffusion coefficient in prediction of grade and prognosis. Radiology 241:839–846
Macdonald DR, Cascino TL, Schold SC Jr et al (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Nagane M, Nozue K, Shimizu S et al (2009) Prolonged and severe thrombocytopenia with pancytopenia induced by radiation-combined temozolomide therapy in a patient with newly diagnosed glioblastoma––analysis of O 6-methylguanine-DNA methyltransferase status. J Neurooncol 92:227–232
Hegi ME, Diserens AC, Gorlia T et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003
Nagane M, Kobayashi K, Ohnishi A et al (2007) Prognostic significance of O6-methylguanine-DNA methyltransferase protein expression in patients with recurrent glioblastoma treated with temozolomide. Jpn J Clin Oncol 37:897–906
Wong ET, Brem S (2008) Antiangiogenesis treatment for glioblastoma multiforme: challenges and opportunities. J Natl Compr Canc Netw 6:515–522
Gerstner ER, Frosch MP, Batchelor TT (2010) Diffusion magnetic resonance imaging detects pathologically confirmed, nonenhancing tumor progression in a patient with recurrent glioblastoma receiving bevacizumab. J Clin Oncol 28:e91–e93
Pope WB, Kim HJ, Huo J et al (2009) Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment. Radiology 252:182–189
Brandsma D, van den Bent MJ (2009) Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol 22:633–638
Levin VA, Bidaut L, Hou P et al (2011) Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys 79:1487–1495
Pope WB, Young JR, Ellingson BM (2011) Advances in MRI assessment of gliomas and response to anti-VEGF therapy. Curr Neurol Neurosci Rep 11:336–344
Ellingson BM, Cloughesy TF, Lai A et al (2012) Quantification of edema reduction using differential quantitative T2 (DQT2) relaxometry mapping in recurrent glioblastoma treated with bevacizumab. J Neurooncol 106:111–119
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972
Pope WB, Lai A, Mehta R et al (2011) Apparent diffusion coefficient histogram analysis stratifies progression-free survival in newly diagnosed bevacizumab-treated glioblastoma. AJNR Am J Neuroradiol 32:882–889
Acknowledgments
This work was supported partially by grants of the Ministry of Health, Labour, and Welfare of Japan (H20-ganrinsyou-ippan-019 and 20shi-4) (to MN). We thank Kuninori Kobayashi, RT (Radiology Section, Kyorin University Hospital) for obtaining MR imaging data.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Nagane, M., Kobayashi, K., Tanaka, M. et al. Predictive significance of mean apparent diffusion coefficient value for responsiveness of temozolomide-refractory malignant glioma to bevacizumab: preliminary report. Int J Clin Oncol 19, 16–23 (2014). https://doi.org/10.1007/s10147-013-0517-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-013-0517-x