Abstract
Background
There are few reports of long-term treatment with docetaxel in castration-resistant prostate cancer (CRPC) because of the limit of a maximum of ten cycles of treatment in TAX327 showing a survival benefit. Therefore, this study, ARD6563, was conducted to evaluate the safety of more than ten cycles of docetaxel in metastatic CRPC.
Methods
We enrolled patients who had received ten cycles of docetaxel in the preceding study, ARD6562. For ARD6563, patients received docetaxel every 3 weeks, at the last dose (70, 60, or 50 mg/m2) received for cycle 10 in ARD6562, with prednisolone 5 mg orally twice daily.
Results
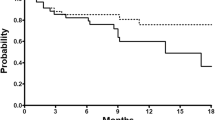
The safety analysis set comprised 15 patients (median age, 64 years; performance status, 0 in 87%) out of 43 patients treated in ARD6562. The median initial dose of docetaxel was 60 mg/m2, and the median number of additional cycles administered was 8 (range, 1–42). The relative dose intensity was 78.0% for docetaxel and 98.0% for prednisolone. Dose reduction was needed in 3 cycles because of grade 3 infection, febrile neutropenia, and grade 2 neuropathy. Administration delay was necessitated in 6 cycles because of grade 1–2 nonhematological toxicities. The major grade 3–4 toxicities were myelosuppression. Five patients who had an observed partial response or stable disease in ARD6562 maintained their clinical response in ARD6563. The study treatment was discontinued in 10 patients because of disease progression and in 4 patients for serious toxicities. There were no treatment-related deaths.
Conclusions
Long-term docetaxel with prednisolone is feasible in selected Japanese patients with CRPC.


Similar content being viewed by others
References
American Cancer Society (2010). Detailed guide: prostate cancer 2009. What are the key statistics about prostate cancer? http://www.cancer.org/docroot/CRI/content/CRI_2_4_1X_What_are_the_key_statistics_for_prostate_cancer_36.asp?sitearea of subordinate document. Accessed March 2011
Ministry of Health, Labor and Welfare (2011) Vital statistics in Japan—the latest trends, p 18. http://www.mhlw.go.jp/english/database/db-hw/dl/81-1a2en.pdf of subordinate document. Accessed March 2011
Suzuki K (2009) Epidemiology of prostate cancer and benign prostatic hyperplasia. JMAJ 52:478–483. http://www.med.or.jp/english/journal/pdf/2009_06/478_483.pdf of subordinate document
Ringel I, Horwitz SB (1991) Studies with RP 56976 (taxotere): a semisynthetic analogue of taxol. J Natl Cancer Inst 83:288–291
Tannock IF, de Wit R, Berry WR et al (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351:1502–1512
Petrylak DP, Tangen CM, Hussain MH et al (2004) Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 351:1513–1520
Chowdhury S, Burbridge S, Harper PG (2007) Chemotherapy for the treatment of hormone-refractory prostate cancer. Int J Clin Pract 61:2064–2070
Goh BC, Lee SC, Wang LZ et al (2002) Explaining interindividual variability of docetaxel pharmacokinetics and pharmacodynamics in Asians through phenotyping and genotyping strategies. J Clin Oncol 20:3683–3690
Clarke SJ, Rivory LP (1999) Clinical pharmacokinetics of docetaxel. Clin Pharmacokinet 36:99–114
Naito S, Tsukamoto T, Koga H et al (2008) Docetaxel plus prednisolone for the treatment of metastatic hormone-refractory prostate cancer: a multicenter phase II trial in Japan. Jpn J Clin Oncol 38:365–372
Chin SN, Wang L, Moore M et al (2010) A review of the patterns of docetaxel use for hormone-resistant prostate cancer at the Princess Margaret Hospital. Curr Oncol 17:24–29
Beer TM, Garzotto M, Henner WD et al (2004) Multiple cycles of intermittent chemotherapy in metastatic androgen-independent prostate cancer. Br J Cancer 91:1425–1427
Miller K, Wuelfing C, Lehmann J et al (2005) Weekly docetaxel plus estramustine for hormone-refractory prostate cancer (HRPC) with intermittent repetition: preliminary results of a multicenter phase II study (AUO AP33/02). J Clin Oncol 23(16S Pt 1 Suppl):406s (abstract 4613)
Beer TM, Ryan CW, Venner PM et al (2008) Intermittent chemotherapy in patients with metastatic androgen-independent prostate cancer: results from ASCENT, a double-blinded, randomized comparison of high-dose calcitriol plus docetaxel with placebo plus docetaxel. Cancer (Phila) 112:326–330
Ansari J, Hussain SA, Zarkar A et al (2008) Docetaxel chemotherapy for metastatic hormone refractory prostate cancer as first-line palliative chemotherapy and subsequent re-treatment: Birmingham experience. Oncol Rep 20:891–896
Soga N, Kato M, Nishikawa K et al (2009) Intermittent docetaxel therapy with estramustine for hormone-refractory prostate cancer in Japanese patients. Int J Clin Oncol 14:130–135
Di Lorenzo G, Buonerba C, Faiella A et al (2011) Phase II study of docetaxel re-treatment in docetaxel-pretreated castration-resistant prostate cancer. BJU Int 107:234–239
de Bono JS, Oudard S, Ozguroglu M et al (2010) Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376:1147–1154
de Bono JS, Logothetis CJ, Molina A et al (2011) Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 364:1995–2005
Acknowledgments
We thank the men who participated in this study. This study was funded by sanofi-aventis KK, and editorial assistance in the preparation of this manuscript was provided by the Asia-Pacific Medical Writing Team (AMRIT) of the sanofi-aventis Group.
Conflict of interest
One of the authors (H.A.) received honoraria (e.g., lecture fees) from sanofi-aventis KK (Tokyo, Japan); the other authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Nishimura, K., Nonomura, N., Hashine, K. et al. Prolonged treatment with three-weekly docetaxel plus daily prednisolone for metastatic castration-resistant prostate cancer: a multicenter, phase II, open-label, non-comparative, extension study in Japan. Int J Clin Oncol 18, 306–313 (2013). https://doi.org/10.1007/s10147-012-0380-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-012-0380-1