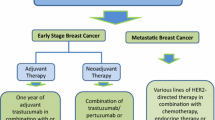
Abstract
HER2-positive tumors account for approximately 18–20% of all breast cancers. These tumors tend to be more aggressive than HER2-negative tumors and are associated with a poorer prognosis. HER2 overexpression, as determined by either 3+ immunohistochemical staining for HER2 protein or HER2 gene amplification by fluorescence in situ hybridization, should be used to select patients for anti-HER2 therapy. Trastuzumab-containing regimens as first-line therapy should be recommended to women with HER2-positive metastatic breast cancer. The continuation of trastuzumab plus capecitabine provided a significant clinical benefit compared with capecitabine alone in women who experienced progression during trastuzumab treatment. An adjuvant trastuzumab-containing regimen should be also recommended to all intermediate- or high-risk women with HER2-positive early breast cancer. Cardiac function should be serially monitored during this treatment. Many anti-HER2 drugs against breast cancer are being developed. The basic mechanisms of their action and resistance emergence are being clarified step by step. Over the mid- or long term, clinical trials comparing these drugs will be conducted until drugs that are clinically effective and easy to use in the true sense survive. Biomarkers are being aggressively searched for concerning individual drugs under development. A position of the “proper drug for the proper patient” will be more firmly established.
Similar content being viewed by others
References
Gusterson BA, Gelber RD, Goldhirsch A et al (1992) Prognostic importance of c-erbB-2 expression in breast cancer. International (Ludwig) Breast Cancer Study Group. J Clin Oncol 10:1049–1056
Wolff AC, Hammond ME, Schwartz JN et al (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25:118–145
Slamon DJ, Leyland-Jones B, Shak S et al (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783–792
Smith I, Procter M, Gelber RD et al (2007) 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet 369:29–36
Romond EH, Perez EA, Bryant J et al (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353:1673–1684
Slamon DJ, Eiermann W, Robert N et al (2005) Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC → T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC → TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2 positive early breast cancer patients: BCIRG 006 study. Breast Cancer Res Treat 94:S5
Geyer CE, Forster J, Lindquist D et al (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355:2733–2743
Lin NU, Carey LA, Liu MC et al (2008) Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 26:1993–1999
Bernard-Marty C, Lebrun F, Awada A et al (2006) Monoclonal antibody-based targeted therapy in breast cancer: current status and future directions. Drugs 66:1577–1591
Agus DB, Gordon MS, Taylor C et al (2005) Phase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancer. J Clin Oncol 23:2534–2543
Rabindran SK, Discafani CM, Rosfjord EC et al (2004) Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res 64:3958–3965
Wong KK, Fracasso PM, Bukowski RM et al (2009) A phase I study with neratinib (HKI-272), an irreversible pan ErbB receptor tyrosine kinase inhibitor, in patients with solid tumors. Clin Cancer Res 15:2552–2558
Viani GA, Afonso SL, Stefano EJ et al (2007) Adjuvant trastuzumab in the treatment of her-2-positive early breast cancer: a meta-analysis of published randomized trials. BMC Cancer 7:153
Perez EA, Suman VJ, Davidson NE et al (2008) Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol 26:1231–1238
Tan-Chiu E, Yothers G, Romond E et al (2005) Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol 23:7811–7819
Gomez HL, Doval DC, Chavez MA et al (2008) Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer. J Clin Oncol 26:2999–3005
Perez EA, Koehler M, Byrne J et al (2008) Cardiac safety of lapatinib: pooled analysis of 3689 patients enrolled in clinical trials. Mayo Clin Proc 83:679–686
(2010) NCCN Clinical Practice Guidelines in Oncology Breast cancer V.2.2010. http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf
Inoue K, Nakagami K, Mizutani M et al (2010) Randomized phase III trial of trastuzumab monotherapy followed by trastuzumab plus docetaxel versus trastuzumab plus docetaxel as first-line therapy in patients with HER2-positive metastatic breast cancer: the JO17360 Trial Group. Breast Cancer Res Treat 119:127–136
Johnston S, Pippen J Jr, Pivot X et al (2009) Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol 27:5538–5546
Kaufman B, Mackey JR, Clemens MR et al (2009) Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol 27:5529–5537
Gelmon KA, Mackey J, Verma S et al (2004) Use of trastuzumab beyond disease progression: observations from a retrospective review of case histories. Clin Breast Cancer 5:52–58 (discussion 59–62)
Bartsch R, Wenzel C, Altorjai G et al (2007) Capecitabine and trastuzumab in heavily pretreated metastatic breast cancer. J Clin Oncol 25:3853–3858
von Minckwitz G, du Bois A, Schmidt M et al (2009) Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a German Breast Group 26/Breast International Group 03–05 study. J Clin Oncol 27:1999–2006
Cortes J, Baselga J, Petrella T et al (2009) Pertuzumab monotherapy following trastuzumab-based treatment: activity and tolerability in patients with advanced HER2-positive breast cancer. J Clin Oncol 27:15s
Piccart-Gebhart MJ, Procter M, Leyland-Jones B et al (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353:1659–1672
Joensuu H, Kellokumpu-Lehtinen PL, Bono P et al (2006) Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med 354:809–820
Garcia JM, Silva JM, Dominguez G et al (1999) Allelic loss of the PTEN region (10q23) in breast carcinomas of poor pathophenotype. Breast Cancer Res Treat 57:237–243
Nagata Y, Lan KH, Zhou X et al (2004) PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 6:117–127
Berns K, Horlings HM, Hennessy BT et al (2007) A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 12:395–402
Christianson TA, Doherty JK, Lin YJ et al (1998) NH2-terminally truncated HER-2/neu protein: relationship with shedding of the extracellular domain and with prognostic factors in breast cancer. Cancer Res 58:5123–5129
Anido J, Scaltriti M, Bech Serra JJ et al (2006) Biosynthesis of tumorigenic HER2 C-terminal fragments by alternative initiation of translation. EMBO J 25:3234–3244
Scaltriti M, Rojo F, Ocana A et al (2007) Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst 99:628–638
Nagy P, Friedlander E, Tanner M et al (2005) Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res 65:473–482
Hubbard SR, Miller WT (2007) Receptor tyrosine kinases: mechanisms of activation and signaling. Curr Opin Cell Biol 19:117–123
Lu Y, Zi X, Zhao Y et al (2001) Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin). J Natl Cancer Inst 93:1852–1857
Le XF, Claret FX, Lammayot A et al (2003) The role of cyclin-dependent kinase inhibitor p27Kip1 in anti-HER2 antibody-induced G1 cell cycle arrest and tumor growth inhibition. J Biol Chem 278:23441–23450
Nahta R, Yuan LX, Zhang B et al (2005) Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res 65:11118–11128
Spector NL, Xia W, Burris H 3rd et al (2005) Study of the biologic effects of lapatinib, a reversible inhibitor of ErbB1 and ErbB2 tyrosine kinases, on tumor growth and survival pathways in patients with advanced malignancies. J Clin Oncol 23:2502–2512
Blackwell KL, Burstein HJ, Storniolo AM et al (2010) Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol 28:1124–1130
Swaby R, Blackwell K, Jiang Z et al (2009) Neratinib in combination with trastuzumab for the treatment of advanced breast cancer: a phase I/II study. J Clin Oncol 27:15s
Vogel CL, Cobleigh MA, Tripathy D et al (2002) Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 20:719–726
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Mukai, H. Treatment strategy for HER2-positive breast cancer. Int J Clin Oncol 15, 335–340 (2010). https://doi.org/10.1007/s10147-010-0107-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-010-0107-0