Abstract
Objective
To evaluate the efficacy and toxicity of third-line gemcitabine monotherapy (Gem) in patients with platinum-resistant advanced urothelial cancer (UC).
Patients and methods
From July 2005 to March 2009, 13 patients were enrolled. All patients had previously received methotrexate, vinblastine, doxorubicin, and cisplatin as first-line therapy. Second-line therapy consisted of paclitaxel/carboplatin (Pca) therapy: paclitaxel (175 mg/m2) followed by carboplatin (area under the curve = 5) was intravenously infused on day 1 of each 21-day cycle. Following Pca failure, Gem was given as third-line treatment: gemcitabine (1,000 mg/m2) was intravenously administered on days 1, 8, and 15 of each 28-day cycle. All patients were eligible for toxicity assessment. Survival curves were produced using the Kaplan–Meier method.
Results
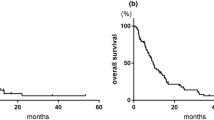
An average of 3.2 Gem cycles (range, 1–8 cycles) were given. Following Gem treatment, overall response rates were 0% CR, 7.7% PR (n = 1), 53.8% SD (n = 7), and 38.5% PD (n = 5). Grade 3–4 toxicities included anemia (31%), neutropenia (31%), and thrombocytopenia (31%). One case experienced grade 3–4 hepatic dysfunction during treatment with Gem. Low-grade alopecia was observed in all 13 patients (100%). Median time to progression and overall survival was 2 and 7.3 months, respectively, following Gem. The 1- and 2-year overall survival rate was 30.8% and 15.3%, respectively, for Gem.
Conclusion
Gem as third-line therapy was performed safely with good tolerability in platinum-resistant advanced UC, even though the efficacy was very limited.


Similar content being viewed by others
References
Saxman SB, Propert KJ, Einhorn LH et al (1997) Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol 15:2564–2569
von der Maase H, Sengelov L, Roberts JT et al (2005) Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 23:4602–4608
Rosenberg JE, Caroll PR, Small FJ (2005) Update on chemotherapy for advanced bladder cancer. J Urol 174:14–20
Pycha A, Grbovic M, Posch B et al (1999) Paclitaxel and carboplatin in patients with metastatic transitional cell cancer of the urinary tract. Urology 53:510–515
Sweeney CJ, Williams SD, Finch DE et al (1999) A phase II study of paclitaxel and ifosfamide for patients with advanced refractory carcinoma of the urothelium. Cancer (Phila) 86:514–518
Tu SM, Hossan E, Amato R et al (1995) Paclitaxel, cisplatin and methotrexate combination chemotherapy is active in the treatment of refractory urothelial malignancies. J Urol 154:1719–1722
Sternberg CN, Calabro F, Pizzocaro G et al (2001) Chemotherapy with an every-2-week regimen of gemcitabine and paclitaxel in patients with transitional cell carcinoma who have received prior cisplatin-based therapy. Cancer (Phila) 92:2993–2998
Soga N, Onishi T, Arima K et al (2007) Paclitaxel carboplatin chemotherapy as a second-line chemotherapy for advanced platinum resistant urothelial cancer in Japanese cases. Int J Urol 14:828–832
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Meluch AA, Greco FA, Burris HA 3rd et al (2001) Paclitaxel and gemcitabine. J Clin Oncol 19:3018–3024
Krege S, Rembrink V, Borgermann C et al (2001) Docetaxel and ifosfamide as second line treatment for patients with advanced or metastatic urothelial cancer after failure of platinum chemotherapy: a phase 2 study. J Urol 165:67–71
Vaughn DJ, Broome CM, Hussain M (2002) Phase II trial of weekly paclitaxel in patients with previously treated advanced urothelial cancer. J Clin Oncol 20:937
Pectasides D, Aravantinos G, Kalofonos H et al (2001) Combination chemotherapy with gemcitabine and ifosfamide as second-line treatment in metastatic urothelial cancer. A phase II trial conducted by the Hellenic Cooperative Oncology Group. Ann Oncol 12:1417–1422
Pollera CF, Ceribelli A, Crecco M et al (1994) Weekly gemcitabine in advanced bladder cancer: a preliminary report from a phase I study. Ann Oncol 5:182–184
von der Maase H (2000) Gemcitabine in locally advanced and/or metastatic bladder cancer. Crit Rev Oncol Hematol 34:175–183
Stadler WM, Kuzel T, Roth B et al (1997) Phase II study of single-agent gemcitabine in previously untreated patients with metastatic urothelial cancer. J Clin Oncol 15:3394–3398
Moore MJ, Tannock IF, Ernst DS et al (1997) Gemcitabine: a promising new agent in the treatment of advanced urothelial cancer. J Clin Oncol 15:3441–3445
Gebbia V, Testa A, Borsellino N et al (1999) Single agent 2′,2′-difluorodeoxycytidine in the treatment of metastatic urothelial carcinoma: a phase II study. Clin Ter 150:11–15
Lorusso V, Pollera CF, Antimi M et al (1998) A phase II study of gemcitabine in patients with transitional cell carcinoma of the urinary tract previously treated with platinum. Italian Co-operative Group on Bladder Cancer. Eur J Cancer 34:1208–1212
Albers P, Siener R, Hartlein M et al (2002) Gemcitabine monotherapy as second-line treatment in cisplatin-refractory transitional cell carcinoma—prognostic factors for response and improvement of quality of life. Onkologie 25:47–52
Akaza H, Naito S, Usami M et al (2007) Efficacy and safety of gemcitabine monotherapy in patients with transitional cell carcinoma after cisplatin-containing therapy: a Japanese experience. Jpn J Clin Oncol 37:201–206
Ecke TH, Gerullis H, Bartel P et al (2009) Palliative chemotherapy with gemcitabine, paclitaxel, and cisplatin as first-line treatment following gemcitabine monotherapy for patients with transitional cell carcinoma of the urothelium. Minerva Urol Nefrol 61:1–8
Conflict of interest statement
No author has any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Soga, N., Kise, H., Arima, K. et al. Third-line gemcitabine monotherapy for platinum-resistant advanced urothelial cancer. Int J Clin Oncol 15, 376–381 (2010). https://doi.org/10.1007/s10147-010-0071-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-010-0071-8