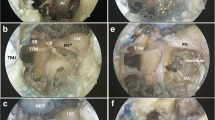
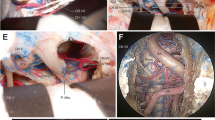
Abstract
Petroclival area lesions are rare, and their surgery is challenging due to the deep location and to the complex relationships between the tumor and the neurovascular structures. The objective is to present a petroclival tumor model simulating the distorted anatomy of a real petroclival lesion and propose its use to practice microsurgical removal while preserving neurovascular structures. Four embalmed cadaver heads were used in this study. An endoscopic endonasal transclival approach was used to access the dura in front of the trigeminal nerve; a pediatric Foley was inserted above the trigeminal nerve and was gradually inflated (one-balloon technique). If a larger tumor model was desired, an additional balloon was placed below the trigeminal nerve (two-balloon technique). A pre-mixed tumor polymer was injected into the petroclival space and allowed to harden to create an implanted tumor. A post-implant CT scan was done to evaluate the location and volume of the implanted artificial tumor. Tumors were subsequently excised via retrosigmoid and anterior petrosal approaches. Six petroclival tumors were successfully developed: three were small (9.41–10.36 ml) and three large (21.05–23.99 ml). During dissection, distorted anatomy created by the tumor model mimicked that of real surgery. We have established a petroclival tumor model with adjustable size which offers opportunities to study the distorted anatomy of the area and that is able to be used as a training tool to practice microsurgical removal of petroclival lesions. The practice dissection of this tumor model can be a bridge between a normal anatomic dissection and real surgery.




Similar content being viewed by others
References
Al-Mefty O, Fox JL, Smith RR (1988) Petrosal approach for petroclival meningiomas. Neurosurgery 22:510–517
Almefty R, Dunn IF, Pravdenkova S, Abolfotoh M, Al-Mefty O (2014) True petroclival meningiomas: results of surgical management. J Neurosurg 120:40–51
Baidya NB, Berhouma M, Ammirati M (2014) Endoscope-assisted retrosigmoid resection of a medium size vestibular schwannoma tumor model: a cadaveric study. Clin Neurol Neurosurg 119:35–38
Bambakidis NC, Kakarla UK, Kim LJ, Nakaji P, Porter RW, Daspit CP, Spetzler RF (2007) Evolution of surgical approaches in the treatment of petroclival meningiomas: a retrospective review. Neurosurgery 61(5 Suppl 2):202–209
Berhouma M, Baidya NB, Ismaïl AA, Zhang J, Ammirati M (2013) Shortening the learning curve in endoscopic endonasal skull base surgery: a reproducible polymer tumor model for the trans-sphenoidal trans-tubercular approach to retro-infundibular tumors. Clin Neurol Neurosurg 115:1635–1641
Bricolo AP, Turazzi S, Talacchi A, Cristofori L (1992) Microsurgical removal of petroclival meningiomas: a report of 33 patients. Neurosurgery 31:813–828
Couldwell WT, Fukushima T, Giannotta SL, Weiss MH (1996) Petroclival meningiomas: surgical experience in 109 cases. J Neurosurg 84:20–28
Flannery TJ, Kano H, Lunsford LD, Sirin S, Tormenti M, Niranjan A, Flickinger JC, Kondziolka D (2010) Long-term control of petroclival meningiomas through radiosurgery. J Neurosurg 112:957–964
Gragnaniello C, Gagliardi F, Chau AM, Nader R, Siu A, Litvack Z, Luca BD, Seex K, Mortini P, Caputy AJ, Al-Mefty O (2014) Intracranial injectable tumor model: technical advancements. J Neurol Surg B Skull Base 75:301–308
Gragnaniello C, Nader R, van Doormaal T, Kamel M, Voormolen EH, Lasio G, Aboud E, Regli L, Tulleken CA, Al-Mefty O (2010) Skull base tumor model. J Neurosurg 113:1106–1111
Ichimura S, Kawase T, Onozuka S, Yoshida K, Ohira T (2008) Four subtypes of petroclival meningiomas: differences in symptoms and operative findings using the anterior transpetrosal approach. Acta Neurochir (Wien) 150:637–645
Kawase T, Shiobara R, Toya S (1991) Anterior transpetrosal-transtentorial approach for sphenopetroclival meningiomas: surgical method and results in 10 patients. Neurosurgery 28:869–875
Kawase T, Shiobara R, Toya S (1994) Middle fossa transpetrosal-transtentorial approaches for petroclival meningiomas. Selective pyramid resection and radicality. Acta Neurochir (Wien) 129:113–120
Little KM, Friedman AH, Sampson JH, Wanibuchi M, Fukushima T (2005) Surgical management of petroclival meningiomas: defining resection goals based on risk of neurological morbidity and tumor recurrence rates in 137 patients. Neurosurgery 56:546–559
Maurer AJ, Safavi-Abbasi S, Cheema AA, Glenn CA, Sughrue ME (2014) Management of petroclival meningiomas: a review of the development of current therapy. J Neurol Surg B Skull Base 75:358–367
Nanda A, Javalkar V, Banerjee AD (2011) Petroclival meningiomas: study on outcomes, complications and recurrence rates. J Neurosurg 114:1268–1277
Natarajan SK, Sekhar LN, Schessel D, Morita A (2007) Petroclival meningiomas: multimodality treatment and outcomes at long-term follow-up. Neurosurgery 60:965–979
Park CK, Jung HW, Kim JE, Paek SH, Kim DG (2006) The selection of the optimal therapeutic strategy for petroclival meningiomas. Surg Neurol 66:160–165
Samii M, Tatagiba M (1992) Experience with 36 surgical cases of petroclival meningiomas. Acta Neurochir (Wien) 118:27–32
Seifert V (2010) Clinical management of petroclival meningiomas and the eternal quest for preservation of quality of life: personal experiences over a period of 20 years. Acta Neurochir (Wien) 152:1099–1116
Spetzler RF, Daspit CP, Pappas CT (1992) The combined supra- and infratentorial approach for lesions of the petrous and clival regions: experience with 46 cases. J Neurosurg 76:588–599
Starke RM, Williams BJ, Hiles C, Nguyen JH, Elsharkawy MY, Sheehan JP (2012) Gamma knife surgery for skull base meningiomas. J Neurosurg 116:588–597
Xu F, Karampelas I, Megerian CA, Selman WR, Bambakidis NC (2013) Petroclival meningiomas: an update on surgical approaches, decision making, and treatment results. Neurosurg Focus 35:E11
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors declare that they have no competing interests.
Additional information
Comments
Jose Alberto Landeiro, Jose Orlando Junior, Rio de Janeiro, Brazil
Several lesions may arise in the petroclival region, and the most frequent is meningioma [1]. By definition, a “true” petroclival meningioma has its origin at the upper two thirds of the clivus medial to the fifth cranial nerve and has distinct clinical and outcome features [1, 6]. In the majority of cases, meningiomas are benign tumors (WHO grade I classification system) [2], and their slow-growing pattern allow them to reach large size before become apparent clinically [7].
The petroclival region contains important neurovascular structures that are frequently involved or displaced by tumor in a variable pattern. Usually, the third and fourth nerves are displaced upwardly, the sixth nerve is frequently surrounded by the tumor, and the basilar artery with its branches may be wrapped [1].
Petroclival meningioma has been described in the early to mid twentieth century as a relentlessly progressive tumor, with ultimately fatal outcomes [12, 13, 14]. In the past decade, Van Havenbergh et al. evaluated the natural history of 21 cases of petroclival meningiomas that were followed for a period of 4 to 10 yeas and found that 76 % of tumors grew radiographically and 50 % of the initially asymptomatic patients developed some kind of cranial nerve deficit [11]. Therefore, these tumors often need treatment [6].
Petroclival meningiomas are formidable surgical challenges attributable to their deep-seated location and proximity and adhesion to cranial nerves, major blood vessels, and the brainstem [3, 4]. Although advances in microsurgery and skull base techniques have been developed, the mortality rate ranges from 0 to 9 % and the incidence of permanent cranial nerve deficits has been shown to vary from 20.3 to 76 % in a number of series [5, 15]. Due to their often benign pathology and the risks associated with operating in this region, there has been much debate over the proper management of these tumors, and with the advent of radiosurgery, many have recommended subtotal removal and subsequent radiosurgery to avoid operative morbidity [7].
Although the use of stereotactic radiosurgery for intracranial meningiomas have been described extensively in the literature, there are few large reports over the treatment of posterior fossa meningiomas and fewer studies include long-term follow-up [8, 9, 10]. Starke et al. have described a large series of petroclival meningioma treated with gamma-knife stereotactic radiosurgery as an adjunct to microsurgery or a primary treatment modality and showed favorable outcomes, especially for smaller tumor, no previous radiation therapy and patients free of symptom [9]. Clival or petrous-based locations are predictive of an increased risk of new or worsening neurological deficit following gamma-knife radiosurgery [8]. Couldwell, Cole, and Al-Mefty have reported aggressive growth of skull base meningioma after failed radiosurgery, and careful extended (exceeding 10 years) follow-up evaluations must be undertaken for all patients after radiosurgery [16].
Almefty et al. have advocated the total resection as a primary goal for petroclival meningioma, if the circumstances allow it, and it was achievable in 76.4 % of their 64 cases and is facilitated by the use of appropriate skull base approaches, with good outcome and functional status. Radiosurgery was considered only for cases where the unresectable residual tumor regrows [6].
The preferred surgical approach for treatment of petroclival meningiomas is still controversial [5]. Bambakidis et al. have considered the retrosigmoid craniotomy as the workhorse surgical approach for most petroclival tumors of any size and reserved the combined transpetrosal approach for tumors that are medial to the internal auditory canal and extend to both middle and posterior cranial fossa [5]. Erkmen, Pravdenkova, and Al-Mefty have proposed an algorithm for selection of the skull base approach requiring careful examination of the preoperative MR imaging, hearing evaluation, and venous anatomy. They proposed the anterior petrosal approach for small petroclival lesions superior to the internal auditory canal; for larger tumors that extend below the internal auditory canal with intact or serviceable hearing, which should undergo a posterior petrosal approach; for large tumors with loss of hearing, which will benefit from the extended exposure provided by a complete petrosectomy; and for those tumors extending across the midline of the clivus or into the anterior cavernous sinus, which require a combined petrosal approach [3, 6].
The authors of this manuscript have showed a brilliant technique in creating similar conditions offered by a petroclival lesion. They describe in a detailed manner how to produce the tumor model simulating the relationship of it with adjacent structures. Although the petroclival meningiomas are the most frequent tumor in the petroclival region, they are rare, accounting for less than 0.15 % of intracranial tumors [1, 6]. Therefore, the laboratory practice of dissection in a tumor model is a formidable way to improve microsurgical skills and surgical outcomes, offering the opportunity to the Neurosurgeon to face and to become familiar with the anatomical distortion created by a petroclival tumor.
References
1) Ramina, R., Aguiar, P.H.P., Tatagiba, M. in: Samii’s essentials in neurosurgery. Springer, New York, NY; 2008; pp 121-135;
2) Louis, DN, Ohgaki H, Wiestler, OD, Cavenee, WK: World Health Organization classification of tumours of the nervous system. IARC, Lyon, 2007;
3) Erkmen K, Pravdenkova S, Al-Mefty O: Surgical management of petroclival meningiomas: factors determining the choice of approach. Neurosurg Focus 2005 Aug 15;19(2):E7;
4) Diluna ML, Bulsara KR: Surgery for petroclival meningiomas: a comprehensive review of outcomes in the skull base surgery era. Skull Base 2010 Sep;20(5):337-42;
5) Xu F, Karampelas I, Megerian CA, Selman WR, Bambakidis NC: Petroclival meningiomas: an update on surgical approaches, decision making, and treatment results. Neurosurg Focus 2013 Dec;35(6):E11;
6) Almefty R, Dunn IF, Pravdenkova S, Abolfotoh M, Al-Mefty O: True petroclival meningiomas: results of surgical management. J Neurosurg 2014 Jan;120(1):40-51;
7) Bambakidis NC, Kakarla UK, Kim LJ, Nakaji P, Porter RW, Daspit CP, Spetzler RF: Evolution of surgical approaches in the treatment of petroclival meningiomas: a retrospective review. Neurosurgery 2007 Nov;61(5 Suppl 2):202-9; discussion 209-11;
8) Starke RM, Nguyen JH, Rainey J, Williams BJ, Sherman JH, Savage J, Yen CP, Sheehan JP: Gamma knife surgery of meningiomas located in the posterior fossa: factors predictive of outcome and remission. J Neurosurg 2011 May;114(5):1399-409;
9) Starke R, Kano H, Ding D, Nakaji P, Barnett GH, Mathieu D, Chiang V, Yu JB, Hess J, McBride HL, Honea N, Lee JY, Rahmathulla G, Evanoff WA, Alonso-Basanta M, Lunsford LD, Sheehan JP: Stereotactic radiosurgery of petroclival meningiomas: a multicenter study. J Neurooncol 2014 Aug;119(1):169-76;
10) Zachenhofer I, Wolfsberger S, Aichholzer M, Bertalanffy A, Roessler K, Kitz K, Knosp E: Gamma-knife radiosurgery for cranial base meningiomas: experience of tumor control, clinical course, and morbidity in a follow-up of more than 8 years. Neurosurgery 2006 Jan;58(1):28-36; discussion 28-36;
11) Van Havenbergh T, Carvalho G, Tatagiba M, Plets C, Samii M: Natural history of petroclival meningiomas. Neurosurgery 2003 Jan;52(1):55-62; discussion 62-4;
12) Castellano F, Ruggiero G: Meningiomas of the posterior fossa. Acta Radiol 104[Suppl]:1–164, 1953;
13) Cherington M, Shneck SA: Clivus meningiomas. Neurology 16:86–92, 1966;
14) Cushing HW, Eisenhardt L: Meningiomas: their classification, regional behaviour, life history and surgical end results. Springfield, Charles C Thomas, 1938;
15) Beniwal M, Bhat DI, Rao N, Bhagavatula ID, Somanna S: Surgical management of petroclival meningiomas: factors affecting early post-operative outcome. Br J Neurosurg 2015 Apr 2:1-6;
16) Couldwell WT, Cole CD, Al-Mefty O: Patterns of skull base meningioma progression after failed radiosurgery. J Neurosurg 2007 Jan;106(1):30-5.
Imad N. Kanaan, Riyadh, Kingdom of Saudi Arabia
The best venue to teach clinical skills, sound judgment, and surgical dexterity to young neurosurgeons is the operating theater (laboratory of highest order) as correctly stated for the clinical ward by William Halstead. Taken into account the complexity of neurosurgical, otological skull-base interventions and patient’s safety, it is a prerequisite for training young neurosurgeons to have rehearsal for their surgical skills at alternative set-up prior to live surgical experience using modern facilities such as navigation system, surgical simulators or attending microsurgical courses, and cadaver dissection workshops using artificial models similar to the one described by Grananiello and validated by the authors. This should be eventually complemented by witness life surgery performed by the great masters in the field. The authors eloquently highlight some of the challenges to be considered such as mixture rate, time between injection and dissection, and proper preparation of the embalmment of the cadaveric head in order to optimise the use of this model. Tactile sensation and tissue feedback remain an issue to be taught at life surgery in their future training.
Atul Goel, Manu Kothari, Mumbai, India
Amongst all things non-clinical, dissection of human cadavers has been now and again viewed as needless, dispensable, and excusable. No wonder then that in advanced academic debates, dissection of a cadaver had been evaluated with points in favor and against. The votes against outnumber those for dissection largely on the grounds of time saved, no bother of cadaveric management, and so on. Even in the home ground of Henry Gray, dissection is on the “decline” (Utting and Willan, 1995) [6].
Be as it may, this presentation wishes to sing the song of the undeniable and overwhelming advantages, benefits, and the joys of cadaveric dissection. We are used to neglecting the seasons, the stars, the spring, sunset, and sunshine. So, what place can there be for such a dispensable exercise as dissection when a CD-ROM can unpeel the human body layer by layer, vessel by vessel, may be one day cell by cell? Our addiction to the computer screen has triumphed over the avoidable exercise of a dissection of “dead-body.” Our alienation from what is real and our fascination with what is a mere shadow play is complete.
The cadaver on dissection table is fully a human being, save that “prana” or “chaitanya (life)” is not permeating it. Otherwise, cell-to-cell and fiber-to-fiber, a live human being and a cadaver are structurally alike. It has been emphasized that dissection is the opportune moment at which students can be inculcated with the values of respect and compassion. A CD or an image (MRI or CT) feeds the eyes, but what of the hands, the touch receptors, the tension receptors, and so on? It cannot provide the dimensions or give the idea of the texture of the artery, the toughness of a tendon, or the resilience of fascia or cartilage. Nothing can replace the hands-on experience that dissection provides. It is only the direct contact with the cadaver that can impress upon the student’s mind the importance of being alive and robust.
Jean Ternel declared. “What is geography to history, anatomy is to medicine.” It described the theater to events. Any knowing of the human body, short of dissection, is to travel the world on a map and to eat the menu card. It is like learning to swim without entering into water. There is no escape for us from feeling, cutting, probing, and dissecting a human being that is lifeless but full of all other mysteries. Robert Boyle of Boyle’s law of gasses aphorized that the human body is a divine mansion in which God has come to stay for a while, and we must know the making and working of it. Dissection is trekking in the wilderness of the human universe. Let dissection be! All textbooks of anatomy, including Gray’s, and all imaging and computer techniques are accessory to dissection but should not, and cannot, ever replace it. We are the inheritors of privileges that have been passed on to us by Sushruta and Vesalius. Let us not fritter our treasures in the name of experience and modernization. Dissection is golden and all the rest is silvery. Once again, let dissection prevail in the learning of surgery.
It is heartening to see the contribution by Dr. Ammirati and his colleagues in designing an artificial tumor model. Although no model can simulate a lifelike situation, it is an attempt to enhance the scope of learning and practicing prior to actually performing on live humans. Anatomical understanding is the key for a surgeon [1-5]. If one can acquire the key, doors can open up. Through this note, I wish to congratulate Dr. Ammirati and his colleagues who have enhanced the scope of anatomical dissections and through their contribution stressed on the importance of preoperative planning and workouts before actually entering the arena of the “living.”
References
1. Goel A (1996) Cavernous sinus: a speculative note. J Clin Neuroscience 3, 281.
2. Goel A (1997) The extradural approach to lesions involving cavernous sinus. Br J Neurosurg 11(2): 134-138.
3. Goel A (1998) Meningeal architecture of the cavernous sinus: clinical and surgical implications (letter). Neurosurgery 42,430-431.
4. Goel A (1998) Impact of arterial relationship on strategy for cavernous sinus tumour surgery. Neurol India 46, 94-101.
5. Goel A, Kothari M, Kobayashi S: Cavernous sinus: a philosophy and anatomy. 147-162 Neurosurgery of complex tumours and vascular lesions’ by Kobayashi S, Goel A, Hongo K, Churchill Livingstone, New York, 1997.
6. Utting M, Willan P (1995) What future for dissection in courses of human topographical anatomy in universities in the UK? Clin Anat. 8(6):414-7.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Endoscopic endonasal transclival approach to implant right artificial petroclival tumor (MPG 21750 kb)
Removal of a right artificial petroclival tumor using a retrosigmoid approach (MPG 22358 kb)
Rights and permissions
About this article
Cite this article
Lee, JS., Tailor, AR., Lamki, T. et al. Petroclival tumor model—technical note and educational implications. Neurosurg Rev 39, 251–258 (2016). https://doi.org/10.1007/s10143-015-0683-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-015-0683-6