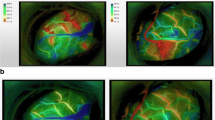
Abstract
We investigated whether postoperative hyperperfusion in moyamoya disease can be predicted using intraoperative laser Doppler flowmetry and/or thermography. A prospective study was conducted on 27 patients (39 hemispheres) with moyamoya disease who underwent superficial temporal artery–middle cerebral artery (STA–MCA) bypass. During surgery, regional cerebral blood flow (rCBF) was measured with a laser Doppler flowmeter and the temperature of the cortical surface was measured with an infrared thermograph. Postoperative hyperperfusion was assessed immediately after surgery based on CBF study under sedation (propofol) as >100% increase in corrected rCBF compared to preoperative values. Postoperative hyperperfusion on CBF was observed in two patients (7.4%). A significant correlation was observed between intraoperative rCBF changes and postoperative rCBF increase (Pearson’s method: r = 0.555, p = 0.0003; simple regression: Y = 1.22X + 3.289, r 2 = 0.308, p = 0.0004). Furthermore, the rCBF changes measured by laser Doppler flowmetry were significantly greater in patients with postoperative hyperperfusion (p = 0.0193) and CHS (p = 0.0193). The present study suggests that intraoperative rCBF measurement using laser Doppler flowmetry may predict a risk of post-EC–IC bypass cerebral hyperperfusion in moyamoya disease.


Similar content being viewed by others
References
Amin-Hanjani S, Du X, Mlinarevich N, Meglio G, Zhao M, Charbel FT (2005) The cut flow index: an intraoperative predictor of the success of extracranial–intracranial bypass for occlusive cerebrovascular disease. Neurosurgery 56(1 Suppl):75–85
Fujimoto S, Toyoda K, Inoue T, Hirai Y, Uwatoko T, Kishikawa K, Yasumori K, Ibayashi S, Iida FM, Okada Y (2004) Diagnostic impact of transcranial color-coded real-time sonography with echo contrast agents for hyperperfusion syndrome after carotid endarterectomy. Stroke 35:1852–1856
Fujimura M, Mugikura S, Kaneta T, Shimizu H, Tominaga T (2009) Incidence and risk factors for symptomatic cerebral hyperperfusion after superficial temporal artery–middle cerebral artery anastomosis in patients with moyamoya disease. Surg Neurol 71:442–447
Fujimura M, Shimizu H, Mugikura S, Tominaga T (2009) Delayed intracerebral hemorrhage after superficial temporal artery–middle cerebral artery anastomosis in a patient with moyamoya disease: possible involvement of cerebral hyperperfusion and increased vascular permeability. Surg Neurol 71:223–227
Horowitz M, Yonas H, Albright AL (1995) Evaluation of cerebral blood flow and hemodynamic reserve in symptomatic moyamoya disease using stable Xenon-CT blood flow. Surg Neurol 44:251–262
Hoshino T, Katayama Y, Sakatani K, Kano T, Murata Y (2006) Intraoperative monitoring of cerebral blood oxygenation and hemodynamics during extracranial–intracranial bypass surgery by a newly developed visible light spectroscopy system. Surg Neurol 65:569–576
Hosoda K, Kawaguchi T, Shibata Y, Kamei M, Kidoguchi K, Koyama J, Fujita S, Tamaki N (2001) Cerebral vasoreactivity and internal carotid artery flow help to identify patients at risk for hyperperfusion after carotid endarterectomy. Stroke 32:1567–1573
Jorgensen L, Schroeder T (1993) Defective cerebrovascular autoregulation after carotid endarterectomy. Eur J Vasc Surg 7:370–379
Kaisti K, Metsahonkala L, Teras M, Oikonen V, Aalto S, Jaaskelainen S, Hinkka S, Scheinin H (2002) Effects of surgical levels of propofol and sevoflurane anesthesia on cerebral blood flow in healthy subjects studied with positron emission tomography. Anesthesiology 96:1358–1370
Kawamata T, Okada Y, Kawashima A, Yoneyama T, Yamaguchi K, Ono Y, Hori T (2009) Post-carotid endarterectomy cerebral hyperperfusion can be prevented by minimizing intraoperative cerebral ischemia and strict postoperative blood pressure control under continuous sedation. Neurosurgery 64:447–454
Kimme P, Ledin T, Sjoberg F (2007) Dose effect of sevoflurane and isoflurane anesthetics on cortical blood flow during controlled hypotension in the pig. Acta Anaesthesiol Scand 51:607–613
Koga H, Mori K, Ono H, Kuwahara M, Matsuse E (1987) Intraoperative regional thermography during surgery for brain tumor. Neurol Med Chir (Tokyo) 27:1033–1038
Komoribayashi N, Ogasawara K, Kobayashi M, Saitoh H, Terasaki K, Inoue T, Ogawa A (2006) Cerebral hyperperfusion after carotid endarterectomy is associated with preoperative hemodynamic impairment and intraoperative cerebral ischemia. J Cereb Blood Flow Metab 26:878–884
Kondo S, Okada Y, Iseki H, Hori T, Takakura K, Kobayashi A, Nagata H (2000) Thermological study of drilling bone tissue with a high-speed drill. Neurosurgery 46:1162–1168
Nambu K, Suzuki R, Hirakawa K (1995) Cerebral blood flow: measurement with xenon-enhanced dynamic helical CT. Radiology 195:53–57
Nussbaum ES, Erickson DL (2000) Extracranial–intracranial bypass for ischemic cerebrovascular disease refractory to maximal medical therapy. Neurosurgery 46:37–43
Ogasawara K, Komoribayashi N, Kobayashi M, Fukuda T, Inoue T, Yamadate K, Ogawa (2005) A neural damage caused by cerebral hyperperfusion after arterial bypass surgery in a patient with moyamoya disease:case report. Neurosurgery 56:E1380
Ogasawara K, Yukawa H, Kobayashi M, Mikami C, Konno H, Terasaki K, Inoue T, Ogawa A (2003) Prediction and monitoring of cerebral hyperperfusion after carotid endarterectomy by using single-photon emission computerized tomography scanning. J Neurosurg 99:504–510
Ohue S, Kumon Y, Kohno K, Watanabe H, Iwata S, Ohnishi T (2008) Postoperative temporary neurological deficits in adults with moyamoya disease. Surg Neurol 69:281–287
Okada Y, Kawamata T, Kawashima A, Hori T (2007) Intraoperative application of thermography in extracranial-intracranial bypass. Neurosurgery 60(4 Suppl 2):362–365
Okada Y, Shima T, Nishida M, Yamane K, Yamada T, Yamanaka C (1998) Effectiveness of superficial temporal artery–middle cerebral artery anastomosis in adult moyamoya disease: cerebral hemodynamics and clinical course in ischemic and hemorrhagic varieties. Stroke 29:625–630
Okada Y, Shima T, Yamane K, Yamanaka C, Kagawa R (1999) Cylindrical or T-shaped silicone rubber stents for microanastomosis —technical note. Neurol Med Chir (Tokyo) 39:55–58
Okudera H, Kobayashi S, Toriyama T (1994) Intraoperative regional and functional thermography during resection of cerebral arteriovenous malformation. Neurosurgery 34:1065–1067
Oshima T, Karasawa F, Satoh T (2002) Effects of propofol on cerebral blood flow and the metabolic rate of oxygen in humans. Acta Anaesthesiol Scand 46:831–835
Ouchi T, Ochiai R, Takeda J, Tsukada H, Kakiuchi T (2006) Combined effects of propofol and mild hypothermia on cerebral metabolism and blood flow in rhesus monkey: a positron emission tomography study. J Anesth 20:208–214
Piepgras D, Morgan M, Sundt TJ, Yanagihara T, Mussman L (1988) Intracerebral hemorrhage after carotid endarterectomy. J Neurosurg 68:532–536
Pinaud M, Lelausque J, Chetanneau A, Fauchoux N, Menegalli D, Souron R (1990) Effects of propofol on cerebral hemodynamics and metabolism in patients with brain trauma. Anesthesiology 73:404–409
Sundt TJ, Sharbrough F, Piepgras D, Kearns T, Messick JJ, O'Fallon W (1981) Correlation of cerebral blood flow and electroencephalographic changes during carotid endarterectomy: with results of surgery and hemodynamics of cerebral ischemia. Mayo Clin Proc 56:533–543
The EC/IC Bypass Study Group (1985) Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. N Engl J Med 313:1191–1200
Uno M, Nakajima N, Nishi K, Shinno K, Nagahiro S (1998) Hyperperfusion syndrome after extracranial–intracranial bypass in a patient with moyamoya disease. Case report. Neuro Med Chir (Tokyo) 38:420–424
van Mook W, Rennenberg R, Schurink G, van Oostenbrugge R, Mess W, Hofman P, de Leeuw P (2005) Cerebral hyperperfusion syndrome. Lancet Neurol 4:877–888
Vandesteene A, Trempont V, Engelman E, Deloof T, Focroul M, Schoutens A, de Rood M (1988) Effect of propofol on cerebral blood flow and metabolism in man. Anaesthesia 43(Suppl):42–43
Woitzik J, Horn P, Vajkoczy P, Schmiedek P (2005) Intraoperative control of extracranial–intracranial bypass patency by near-infrared indocyanine green videoangiography. J Neurosurg 102:692–698
Yamaguchi K, Kawamata T, Kawashima A, Hori T, Okada Y (2010) Incidence and predictive factors of cerebral hyperperfusion after extracranial–intracranial bypass for occlusive cerebrovascular diseases. Neurosurgery 67:1548–1554
Yonas H, Wolfson SK Jr, Gur D, Latchaw RE, Good WF, Leanza R, Jackson DL, Jannetta PJ, Reinmuth OM (1984) Clinical experience with the use of xenon-enhanced CT blood flow mapping in cerebral vascular disease. Stroke 15:443–450
Acknowledgement
This study was supported by research funds of Department of Neurosurgery, Tokyo Women’s Medical University.
Disclosure
The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article
Conflict of Interest
All authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Yasuhiro Yonekawa, Zurich, Switzerland
Kawamata et al. have shown an elegant method to predict a risk of postoperative hyperperfusion after STA-MCA bypassfor Moyamoya disease by using laser Dopplerflowmetryintraoperatively at the time of EC-IC bypass surgery, what could be confirmed by postoperative Xe-CT scan-|rCBF measurement. This simple method would contribute to the management of postoperative hyperperfusion, although its occurrence has been reported to be rather infrequent but can be associated with considerable sequelae.
By the way, it was my little surprise that intraoperative thermography change did not correlate with postoperative rCBF change. This might be due to inherent methodological problems namelythis can be delicately influenced byroom temperature and/or heat produced by the light of operating microscope. I would be interested for the purpose to use the Peltier stack which would have less above mentioned problems and its usefulnessfulness was very impressive at the time of parent artery temporary occlusionalong with simplicity of its handling in aneurysm surgery (1).
References
1. Ogata N, Fournier JY, Imhof HG, Yonekawa Y: Thermal diffusion blood flow monitoring during aneurysm surgery. ActaNeurochir (Wien) 138: 726–731, 1996
Rights and permissions
About this article
Cite this article
Kawamata, T., Kawashima, A., Yamaguchi, K. et al. Usefulness of intraoperative laser Doppler flowmetry and thermography to predict a risk of postoperative hyperperfusion after superficial temporal artery–middle cerebral artery bypass for moyamoya disease. Neurosurg Rev 34, 355–362 (2011). https://doi.org/10.1007/s10143-011-0331-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-011-0331-8