Abstract
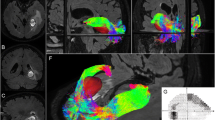
In this paper we report our experience with diffusion-weighted imaging (DWI) for optic radiation (OR) visualization during resection of tumors. We hypothesize that intraoperative OR visualization helps to maintain patients’ visual fields. DWI studies were performed together with T1-weighted postcontrast magnetic resonance imaging (MRI) in four patients with lesions in or adjacent to the OR (glioblastoma, oligo-astrocytoma, cavernoma, and metastasis; n=1 each). The OR was identified from one of six DWI data acquisitions, segmented and reconstructed three-dimensionally. The image data were neuronavigationally transferred into the operative field, and provided the neurosurgeon with information on lesion site and adjacent OR localization. Preoperative and postoperative neuroophthalmological testing included, among others, perimetry to define the value of diffusion-weighted image guidance during OR lesion resection. Three lesions were removed completely. In one case, low-grade tumor parts infiltrating the OR were intentionally left. No persistent visual field deficits were induced. In one patient, a transient homonymous hemianopia attributable to postoperative swelling completely resolved under steroid medication. The authors conclude that intraoperative OR visualization, realized by neuronavigationally displayed DWI data, might prove to be helpful to maintain patients’ visual fields.





Similar content being viewed by others
References
Axer H, Berks G, Keyserlingk DG (2000) Visualization of nerve fiber orientation in gross histological sections of the human brain. Microsc Res Tech 51:481–492
Black P, Jaaskelainen J, Chabrerie A, Golby A, Gugino L (2002) Minimalist approach: functional mapping. Clin Neurosurg 49:90–102
Bürgel U, Schormann T, Schleicher A, Zilles K (1999) Mapping of histologically identified long fiber tracts in human cerebral hemispheres to the MRI volume of a reference brain: position and spatial variability of the optic radiation. NeuroImage 10:489–499
Coenen VA, Krings T, Mayfrank L, Polin RS, Reinges MHT, Thron A, Gilsbach JM (2001) 3D-visualization of the pyramidal tract in a neuronavigation system during brain tumor surgery: first experiences and technical note. Neurosurgery 49:86–93
Coenen VA, Krings T, Axer H, Weidemann J, Kränzlein H, Hans FJ, Thron A, Gilsbach JM, Rohde V (2003) Intraoperative three-dimensional visualization of the pyramidal tract in a neuronavigation system (PTV) reliably predicts true position of principle motor pathways. Surg Neurol 60:381–390
Duffau H, Velut S, Mitchell MC, Gatignol P, Capelle L (2004) Intra-operative mapping of the subcortical visual pathways using direct electrical stimulations. Acta Neurochir (Wien) 146:265–270
Ebeling U, Reulen HJ (1988) Neurosurgical topography of the optic radiation in the temporal lobe. Acta Neurochir 92:29–36
Jensen I, Seedorf HH (1976) Temporal lobe epilepsy and neuroopthalmology: ophthalmological findings in 74 temporal lobe resected patients. Acta Ophthalmol 54:827–841
Kitajima M, Korogi Y, Takahashi M, Eto K (1996) MR signal intensity of the optic radiation. Am J Neuroradiol 17:1379–1383
Klingler J (1948) Die makroskopische Anatomie der Ammonsformation. In: Denkschriften der Schweizerischen Naturforschenden Gesellschaft. Gebrüder Fretz, Zürich
Lantz Tv, Wachsmuth W (1985–2004) Praktische Anatomie. Kopf—übergeordnete Systeme. Erster Band (1a). Springer, Berlin Heidelberg New York
Lazar M, Alexander AL (2003) An error analysis of white matter tractography methods: synthetic diffusion tensor field simulations. NeuroImage 20:1140–1153
Makris N, Worth AJ, Sorensen AG, Papadimitriou GM, Wu O, Rees TG, Wedeen VJ, Davis TL, Stakes JW, Caviness VS, Kaplan E, Rose BR, Pandya DN, Kennedy DN (1997) Morphometry of in vivo human white matter association pathways with diffusion-weighted magnetic resonance imaging. Ann Neurol 42:951–962
Moller AR (1995) Intraoperative neurophysiologic monitoring. Harwood Academic, Luxembourg
Möller-Hartmann W, Krings T, Coenen VA, Mayfrank L, Weidemann J, Kränzlein H, Thron A (2002) Preoperative assessment of motor cortex and pyramidal tracts in central cavernoma employing functional and diffusion-weighted magnetic resonance imaging. Surg Neurol 58:302–308
Nakada T, Nakayama N, Fujii Y, Kwee IL (1999) Clinical application of three-dimensional anisotropy contrast magnetic resonance axonography: a technical note. J Neurosurg 90:791–795
Nieuwenhuys R, Voogd J, van Huijzen C (1991) The human central nervous system—a synopsis and atlas. Springer, Berlin Heidelberg New York
Nimsky C, Gansland O, Cerny S, Hastreiter P, Greiner G, Fahlbusch R (2000) Quantification of, visualization of, and compensation for brain shift using intraoperative magnetic resonance imaging. Neurosurgery 47:1070–1080
Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G (1996) Diffusion tensor MR imaging of the human brain. Radiology 201:637–648
Türe U, Yasargil G, Friedman AH, Al-Mefty O (2000) Fiber dissection technique: lateral aspect of the brain. Neurosurgery 47:417–427
Wandell BA, Wade AR (2003) Functional imaging of the visual pathways. Neurol Clin 21:414–43
Werring DJ, Parker GJM, Miller DH, Thompson AJ, Barker GJ (1999) A direct demonstration of both structure and function in the visual system: combining diffusion tensor imaging with functional magnetic resonance imaging. NeuroImage 9:352–361
Wieshmann UC, Symms MR, Clark CA, Lemieux L, Franconi F, Parker GJ, Barker Gj, Shorvon SD (1999) Wallerian degeneration in the optic radiation after temporal lobectomy demonstrated in vivo with diffusion tensor imaging. Epilepsia 40:1155–1158
Wieshmann UC, Symms MR, Parker GJM, Clark CA, Barker GJ, Shorvon JM (2000) Diffusion tensor imaging demonstrates deviation of fibres in normal appearing white matter adjacent to a brain tumor. J Neurol Neurosurg Psychiatry 68:501–503
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coenen, V.A., Huber, K.K., Krings, T. et al. Diffusion-weighted imaging-guided resection of intracerebral lesions involving the optic radiation. Neurosurg Rev 28, 188–195 (2005). https://doi.org/10.1007/s10143-005-0385-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-005-0385-6