Abstract
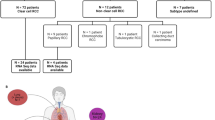
Metabolic reprogramming is essential for establishing the tumor microenvironment (TME). Glutamine has been implicated in cancer metabolism, but its role in clear cell renal carcinoma (ccRCC) remains unknown. Transcriptome data of patients with ccRCC and single-cell RNA sequencing (scRNA-seq) data were obtained from The Cancer Genome Atlas (TCGA, 539 ccRCC samples and 59 normal samples) database and GSE152938 (5 ccRCC samples). Differentially expressed genes related to glutamine metabolism (GRGs) were obtained from the MSigDB database. Consensus cluster analysis distinguished metabolism-related ccRCC subtypes. LASSO-Cox regression analysis was used to construct a metabolism-related prognostic model. The ssGSEA and ESTIMATE algorithms evaluated the level of immune cell infiltration in the TME, and the immunotherapy sensitivity score was obtained from TIDE. Cell–cell communication analysis was used to observe the distribution and effects of the target genes in the cell subsets. An image genomics model was constructed using imaging feature extraction and a machine learning algorithm. Results: Fourteen GRGs were identified. Overall survival and progression-free survival rates were lower in metabolic cluster 2, compared with those in cluster 1. The matrix/ESTIMATE/immune score in C1 decreased, but tumor purity in C2 increased. Immune cells were more active in the high-risk group, in which CD8 + T cells, follicular helper T cells, Th1 cells, and Th2 cells were significantly higher than those in the low-risk group. The expression levels of immune checkpoints were also significantly different between the two groups. RIMKL mainly appeared in epithelial cells in the single-cell analysis. ARHGAP11B was sparsely distributed. The imaging genomics model proved effective in aiding with clinical decisions. Glutamine metabolism plays a crucial role in the formation of immune TMEs in ccRCC. It is effective in differentiating the risk and predicting survival in patients with ccRCC. Imaging features can be used as new biomarkers for predicting ccRCC immunotherapy.









Similar content being viewed by others
Data availability
Any data and R scripts in this study can be obtained from the corresponding author upon reasonable request. The final manuscript has been read and approved by all the authors. In this study, publicly available datasets were analyzed. These data are available from TCGA (https://portal.gdc.cancer.gov/) and GEO (https://www.ncbi.nlm.nih.gov/) databases (Data Release 33.0—May 03, 2022).
Change history
15 May 2024
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s10142-024-01385-0
References
Altman BJ, Stine ZE, Dang CV (2016) From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer 16(10):619–634
Anderson KG, Stromnes IM, Greenberg PD (2017) Obstacles posed by the tumor microenvironment to T cell activity: a case for synergistic therapies. Cancer Cell 31(3):311–325
Angeli I (2004) A consistent set of nuclear rms charge radii: properties of the radius surface r (n, z). At Data Nucl Data Tables 87(2):185–206
Beloribi-Djefaflia S, Vasseur S, Guillaumond F (2016) Lipid metabolic reprogramming in cancer cells. Oncogenesis 5(1):e189-e
Boroughs LK, DeBerardinis RJ (2015) Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol 17(4):351–359
Bryant KL, Mancias JD, Kimmelman AC, Der CJ (2014) Kras: Feeding pancreatic cancer proliferation. Trends Biochem Sci 39(2):91–100
Buchbinder EI, Desai A (2016) Ctla-4 and Pd-1 Pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol 39(1):98
Chen X, Zhou Z, Hannan R, Thomas K, Pedrosa I, Kapur P et al (2018) Reliable gene mutation prediction in clear cell renal cell carcinoma through multi-classifier multi-objective radiogenomics model. Phys Med Biol 63(21):215008
DeBerardinis RJ, Cheng T (2010) Q’s next: the diverse functions of glutamine in metabolism. Cell Biol Cancer Oncog 29(3):313–324
Edwards DN, Ngwa VM, Raybuck AL, Wang S, Hwang Y, Kim LC et al (2021) Selective glutamine metabolism inhibition in tumor cells improves antitumor T lymphocyte activity in triple-negative breast cancer. J Clin Investig 131(4):e140100
Fan T, Sun G, Sun X, Zhao L, Zhong R, Peng Y (2019) Tumor energy metabolism and potential of 3-bromopyruvate as an inhibitor of aerobic glycolysis: implications in tumor treatment. Cancers 11(3):317
Faubert B, Solmonson A, DeBerardinis RJ (2020) Metabolic reprogramming and cancer progression. Science 368(6487):eaaw5473
Gaglio D, Metallo CM, Gameiro PA, Hiller K, Danna LS, Balestrieri C et al (2011) Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Mol Syst Biol 7(1):523
Gerriets VA, Rathmell JC (2012) Metabolic pathways in T cell fate and function. Trends Immunol 33(4):168–173
Hänzelmann S, Castelo R, Guinney J (2013) Gsva: gene set variation analysis for microarray and Rna-Seq data. BMC Bioinforma 14(1):1–15
Hosios AM, Hecht VC, Danai LV, Johnson MO, Rathmell JC, Steinhauser ML et al (2016) Amino acids rather than glucose account for the majority of cell mass in proliferating mammalian cells. Dev Cell 36(5):540–549
Hwang B, Lee JH, Bang D (2018) Single-cell Rna sequencing technologies and bioinformatics pipelines. Exp Mol Med 50(8):1–14
Jin S, Guerrero-Juarez CF, Zhang L, Chang I, Ramos R, Kuan C-H et al (2021) Inference and analysis of cell-cell communication using Cellchat. Nat Commun 12(1):1–20
Jones RG, Thompson CB (2009) Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes Dev 23(5):537–548
Kamphorst JJ, Nofal M, Commisso C, Hackett SR, Lu W, Grabocka E et al (2015) Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Can Res 75(3):544–553
Koelwyn GJ, Quail DF, Zhang X, White RM, Jones LW (2017) Exercise-dependent regulation of the tumour microenvironment. Nat Rev Cancer 17(10):620–632
Koppenol WH, Bounds PL, Dang CV (2011) Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer 11(5):325–337
Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, Van Stiphout RG, Granton P et al (2012) Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 48(4):441–446
Leone RD, Zhao L, Englert JM, Sun I-M, Oh M-H, Sun I-H et al (2019) Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science 366(6468):1013–1021
Li Z, Zhang H (2016) Reprogramming of glucose, fatty acid and amino acid metabolism for cancer progression. Cell Mol Life Sci 73(2):377–392
Li Z, Sun C, Qin Z (2021) Metabolic reprogramming of cancer-associated fibroblasts and its effect on cancer cell reprogramming. Theranostics 11(17):8322
Lian X, Yang K, Li R, Li M, Zuo J, Zheng B et al (2022) Immunometabolic rewiring in tumorigenesis and anti-tumor immunotherapy. Mol Cancer 21(1):1–17
Lieu EL, Nguyen T, Rhyne S, Kim J (2020) Amino acids in cancer. Exp Mol Med 52(1):15–30
Lin X, Xiao Z, Chen T, Liang SH, Guo H (2020) Glucose metabolism on tumor plasticity, diagnosis, and treatment. Front Oncol 10:317
Liu Y, Yang L, An H, Chang Y, Zhang W, Zhu Y et al (2015) High expression of solute carrier family 1, member 5 (Slc1a5) is associated with poor prognosis in clear-cell renal cell carcinoma. Sci Rep 5(1):1–10
Martínez-Reyes I, Chandel NS (2021) Cancer metabolism: looking forward. Nat Rev Cancer 21(10):669–680
Mayakonda A, Lin D-C, Assenov Y, Plass C, Koeffler HP (2018) Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res 28(11):1747–1756
Neu J, DeMarco V, Li N (2002) Glutamine: clinical applications and mechanisms of action. Curr Opin Clin Nutr Metab Care 5(1):69–75
Pathania D, Millard M, Neamati N (2009) Opportunities in discovery and delivery of anticancer drugs targeting mitochondria and cancer cell metabolism. Adv Drug Deliv Rev 61(14):1250–1275
Prior F, Smith K, Sharma A, Kirby J, Tarbox L, Clark K et al (2017) The public cancer radiology imaging collections of the cancer imaging archive. Sci Data 4:170124. https://doi.org/10.1038/sdata.2017.124
Rabinovich GA, Gabrilovich D, Sotomayor EM (2007) Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol 25:267
Reinfeld BI, Madden MZ, Wolf MM, Chytil A, Bader JE, Patterson AR et al (2021) Cell-programmed nutrient partitioning in the tumour microenvironment. Nature 593(7858):282–288
Simonaggio A, Epaillard N, Pobel C, Moreira M, Oudard S, Vano Y-A (2021) Tumor microenvironment features as predictive biomarkers of response to immune checkpoint inhibitors (Ici) in metastatic clear cell renal cell carcinoma (Mccrcc). Cancers 13(2):231
Smaldone MC, Chen DY, Jian QY, Plimack ER (2012) Potential role of 124i-Girentuximab in the presurgical diagnosis of clear-cell renal cell cancer. Biol: Targets Ther 6:395
Wu G, Bazer FW, Dai Z, Li D, Wang J, Wu Z (2014) Amino acid nutrition in animals: protein synthesis and beyond. Annu Rev Anim Biosci 2(1):387–417
Yang M, Soga T, Pollard PJ (2013) Oncometabolites: linking altered metabolism with cancer. J Clin Investig 123(9):3652–3658
Zhu L, Zhu X, Wu Y (2022) Effects of glucose metabolism, lipid metabolism, and glutamine metabolism on tumor microenvironment and clinical implications. Biomolecules 12(4). https://doi.org/10.3390/biom12040580
Acknowledgements
The authors thank the participants and staff for their contribution.
Author information
Authors and Affiliations
Contributions
Conceptualization: Haote Liang; data curation: Keming Wu; formal analysis: Rongrong Wu; supervision and validation: Hongde Chen; other authors contributed to the manuscript.
Corresponding author
Ethics declarations
Consent for publication
All the authors consented to the publication of this study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1007/s10142-024-01385-0
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, H., Wu, K., Wu, R. et al. RETRACTED ARTICLE: Renal enhanced CT images reveal the tandem mechanism between tumor cells and immunocytes based on bulk/single-cell RNA sequencing. Funct Integr Genomics 23, 88 (2023). https://doi.org/10.1007/s10142-023-01011-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10142-023-01011-5