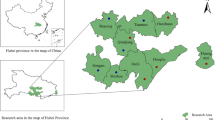
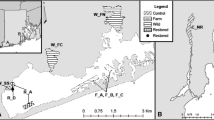
Abstract
The mangrove oyster (Crassostrea gasar) is Brazil’s second most cultured species and presents a high potential for aquaculture. However, artificial selection in a highly fecund species and significant variance in reproductive success can result in the loss of genetic diversity and increases the inbreeding rate, especially in cultivated populations. In this study, we investigated the genetic structure and diversity of C. gasar in wild and cultivated populations using 14 microsatellites. Spatial genetic comparisons revealed the existence of two main genetic groups of C. gasar, one comprising the population in cultivation and the other formed by wild populations along the southern and southeastern Brazilian coasts. Although no common genetic structure exists among wild populations, it is possible to observe a distribution gradient based on discriminant analysis of principal components consistent with their geographic distribution. However, it is insufficient to differentiate them genetically. Despite artificial reproduction, the genetic diversity values of the cultivated population remained relatively high and did not show a reduction. Therefore, monitoring the cultivated population and establishing reference values for genetic diversity will allow the adoption of strategies both for the viability of the cultivated population and the management of wild populations.



Similar content being viewed by others
Availability of Data and Materials
The data that support the findings of this study (genotypes) are available from the B.M.S.S. upon reasonable request.
References
Appleyard SA, Ward RD (2006) Genetic diversity and effective population size in mass selection lines of Pacific oyster (Crassostrea gigas). Aquaculture 254:148–159
Baldan AP, Bendhack F (2009) Sustainable mariculture in Paraná coast, Brazil: updates and perspectives. Cienc Anim Bras 491–497
Baldez R, do SC, Melo MAD, Sampaio I, Tagliaro CH, (2016) Novel microsatellite markers for Brazilian mangrove oysters (Crassostrea gasar) and their cross-amplification in Crassostrea rhizophorae. Braz Arch Biol Technol 59:1–5
Boehs G, Luz MDSA, De Andrade, VRD (2019) Molecular identification of cryptic species of oysters (genus Crassostrea Sacco, 1897) in the northeast atlantic coast of Brazil. Bol Inst Pesca 45(2):2–7
Brunetto LJ, de Gomes CHA, M, Ramos C de O et al (2020) The effect of density on the cultivation of the native mangrove oyster Crassostrea tulipa (Lamarck, 1819). Lat Am J Aquat Res 48:855–868
Carpenter KE, De Angelis N (2016) The living marine resources of the Western Central Atlantic. Volume 1: Introduction, mollusks, crustaceans, hagfishes, sharks, batoid fishes and chimaeras. Rome: FAO. https://www.fao.org/3/y4160e/y4160e00.htm
Cavaleiro NP, Solé-Cava AM, Lazoski C, Cunha HA (2013) Polymorphic microsatellite loci for two Atlantic oyster species: Crassostrea rhizophorae and C. gasar. Mol Biol Rep 40:7039–7043
Chen N, Luo X, Lu C et al (2017) Effects of artificial selection practices on loss of genetic diversity in the Pacific abalone, Haliotis discus hannai. Aquac Res 48:4923–4933
Christo SW et al (2006) Reproductive period of Crassostrea Rhizophorae ( Guilding , 1828 ) and Crassostrea brasiliana Stable. II(39):1215–1218
Dautzenberg (1891) Mémoires de la Société zoologique de France. Au Siege de la Société, Paris. https://www.biodiversitylibrary.org/item/38546#page/24/mode/1up
De Melo CMR, Durland E, Langdon C (2016) Improvements in desirable traits of the Pacific oyster, Crassostrea gigas, as a result of five generations of selection on the West Coast, USA. Aquaculture 460:105–115
Do C, Waples RS, Peel D et al (2014) NeEstimator v2: re-implementation of software for the estimation of contemporary effective population size (Ne) from genetic data. Mol Ecol Resour 14:209–214
Earl DA, VonHoldt BM (2012) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour 4:359–361
Eding H (2002) Conservation of genetic diversity: assessing genetic variation using marker estimated kinships. Wageningen Univ Res 2002. https://www.proquest.com/docview/2560073149?pq-origsite=gscholar&fromopenview=true
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software structure: a simulation study. Mol Ecol 14:2611–2620
Evans F, Matson S, Brake J, Langdon C (2004) The effects of inbreeding on performance traits of adult Pacific oysters (Crassostrea gigas). Aquaculture 230:89–98
Excoffier L, Lischer HEL (2010) Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour 10:564–567
Falush D, Stephens M, Pritchard JK (2003) Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164:1567–1587
Frankham R (1996) Relationship of genetic variation to population size in wildlife. Conserv Biol 10:1500–1508
Galvão MSN, Hilsdorf AWS (2015) Assessing the genetic diversity of the mangrove oyster Crassostrea rhizophorae (Bivalvia, Ostreidae) by microsatellite markers in southeastern Brazil. Mar Biol Res 11:944–954
Galvão MSN, Pereira OM, Machado IC, Henriques MB (2000) Aspectos reprodutivos da ostra Crassostrea brasiliana de manguezais doa estuário de Cananéia, SP (25ºS; 48ºW). Bol Inst Pesca, São Paulo 26:147–162
Galvão MSN, Pereira OM, Hilsdorf AWS (2013) Molecular identification and distribution of mangrove oysters (Crassostrea) in an estuarine ecosystem in southeast Brazil: implications for aquaculture and fisheries management. Aquacul Res 44:1589–1601
Galvao MS, Pereira OM, Machado IC et al (2009) Performance of juvenile culture of the mangrove oyster Crassostrea sp. in suspended lanterns in subtidal zone of the Cananéia estuary and Itagua bay, Ubatuba (São paulo state, brazil). Bol Inst Pesca 35:401–411
Gjedrem T, Baranski M (2009) Selective breeding in aquaculture: an introduction. Springer, Dordrecht. https://link.springer.com/book/10.1007/978-90-481-2773-3
Goudet J (2002) FSTAT (version 2.9.3.2): a computer program to calculate F-statistics. J Hered 86(6):485–486
Hellberg ME, Burton RS, Neigel JE, Palumbi SR (2002) Genetic assessment of connectivity among marine populations. Bull Mar Sci 70(1), 273–290
Ignacio BL, AbsherTM Lazoski C, Solé-Cava AM (2000) Genetic evidence of the presence of twospecies of Crassostrea (Bivalvia: Ostreidae) on the coast of Brazil. Mar Biol 136:987–991
In VV, O’Connor W, Dove M, Knibb W (2016) Can genetic diversity be maintained across multiple mass selection lines of Sydney rock oyster, Saccostrea glomerata despite loss within each? Aquaculture 454:210–216
Jombart T, Bateman A (2008) adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 24:1403–1405
Jombart T, Devillard S, Balloux F (2010) Discriminant analysis of principal components: a new method for the analysis of genetically structured populations. BMC Genet 11:1–15
Kassambara A (2023) ggpubr R Package: ggplot2-based publication ready plots. Datanovia 1–188
Langdon C, Evans F, Jacobson D, Blouin M (2003) Yields of cultured Pacific oysters Crassostrea gigas Thunberg improved after one generation of selection. Aquaculture 220:227–244
Lapègue S, Boutet I, Leitão A et al (2002) Trans-atlantic distribution of a mangrove oyster species revealed by 16S mtDNA and karyological analyses. Biol Bull 202:232–242
Lazoski C, Gusmão J, Boudry P et al (2011) Phylogeny and phylogeography of Atlantic oyster species: evolutionary history, limited genetic connectivity and isolation by distance. Mar Ecol Prog Ser 426:197–212
Leberg PL (2002) Estimating allelic richness: effects of sample size and bottlenecks. Mol Ecol 11:2445–2449
Legat JFA, Puchnick-Legat A, Fogaça FHS et al (2017) Growth and survival of bottom oyster Crassostrea gasar cultured in the northeast and south of brazil. Bol Inst Pesca 43:172–184
Lind CE, Evans BS, Knauer J et al (2009) Decreased genetic diversity and a reduced effective population size in cultured silver-lipped pearl oysters (Pinctada maxima). Aquaculture 286:12–19
Lopes TS, Streit DP, Ribeiro RP et al (2009) Genetic diversity of Colossoma macropomum broodstocks. Arq Bras Med Vet e Zootec 61:728–735
Mateus JC, Eding H, Penedo MCT, Rangel-Figueiredo MT (2004) Contributions of Portuguese cattle breeds to genetic diversity using marker-estimated kinships. Anim Genet 35:305–313
Miller PA, Elliott NG, Koutoulis A et al (2012) Genetic diversity of cultured, naturalized, and native pacific oysters, Crassostrea gigas, determined from multiplexed microsatellite markers. J Shellfish Res 31:611–617
Nascimento IA (1991) Crassostrea rhizophorae (Guilding) and C. brasiliana (Lamarck) in South and Central America. In: Menzel RW (ed) Estuarine and marine bivalve mollusk culture. CRC Press, Boston 125–134
Palumbi SR (1994) Genetic divergence, reproductive isolation, and marine speciation. Annu Rev Ecol Syst 25:547–572
Peakall R, Smouse PE (2012) GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 28:2537
Pereira OM, Henriques MB, Machado IC (2003) Estimativa da curva de crescimento da ostra Crassostrea brasiliana em bosques de mangue e proposta para sua extração ordenada no estuário de Cananéia, SP, Brasil. Bol Inst Pesca 29:19–28
Pie MR, Ribeiro, RO, Boeger WA et al (2006) A simple PCR‐RFLP method for the discrimination of native and introduced oyster species (Crassostrea brasiliana, C. rhizophorae and C. gigas; Bivalvia: Ostreidae) cultured in Southern Brazil. Aquacul Res 37(15):1598–1600
Pritchard JK, Stephens M, Rosenberg NA, Donnelly P (2000) Association mapping in structured populations. Am J Hum Genet 67:170–181
R Core Team (2017) R: a language and environment for statistical computing.
Raymond M, Rousset F (1995) GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Schuelke M (2000) An economic method for the fluorescent labeling of PCR fragments. Nat Biotechnol 18:233–234
Shaklee JB, Bentzen P (1998) Genetic identification of stocks of marine fish and shellfish. Bull Mar Sci 62:589–621
Shanks AL (2009) Pelagic larval duration and dispersal distance revisited. Biol Bull 216:373–385
Shanks AL, Grantham BA, Carr MH (2003) Propagule dispersal distance and the size and spacing of marine reserves. Ecol Appl 13:159–169
Silva TDF (2015) Identidade e diversidade genética de espécies de ostras nativas no Estado de Sergipe. [Masters dissertation, Universidad Federal de Sergipe]. Federal University of Sergipe Repository. https://ri.ufs.br/bitstream/riufs/4488/1/THOMAZ_FRANCA_SILVA.pdf
Silveira R, Silva FC, Gomes CHM et al (2011) Larval settlement and spat recovery rates of the oyster Crassostrea brasiliana (Lamarck, 1819) using different systems to induce metamorphosis. Braz J Biol 71:557–562
Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) micro-checker: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Varela ES, Beasley CR, Schneider H et al (2007) Molecular phylogeny of mangrove oysters (Crassostrea) from Brazil. J Molluscan Stud 73:229–234
Varney RL, Wilbur AE (2020) Analysis of genetic variation and inbreeding among three lines of hatchery-reared Crassostrea virginica broodstock. Aquaculture 527:735452
Waples RS (2006) A bias correction for estimates of effective population size based on linkage disequilibrium at unlinked gene loci. Conserv Genet 72:167–184
Waples RS, Do C (2008) LDNE: a program for estimating effective population size from data on linkage disequilibrium. Mol Ecol Resour 8:753–756
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358
Xu L, Li Q, Xu C et al (2019) Genetic diversity and effective population size in successive mass selected generations of black shell strain Pacific oyster (Crassostrea gigas) based on microsatellites and mtDNA data. Aquaculture 500:338–346
Yu H, Li Q (2007) Genetic variation of wild and hatchery populations of the Pacific oyster Crassostrea gigas assessed by microsatellite markers. J Genet Genomics 34:1114–1122
Zhong X, Feng D, Yu H et al (2016) Genetic variation and breeding signature in mass selection lines of the pacific oyster (Crassostrea gigas) assessed by SNP markers. PLoS ONE 11:1–13
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)-Finance Code 001 to the Federal University of Santa Catarina and by the Concelho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). The authors also thank the CNPq for the Research Productivity scholarship awarded to C.M.R. de Melo and the FAPESC for the scholarship to B.M.S. Scaranto.
Author information
Authors and Affiliations
Contributions
Bianca Maria Soares Scaranto: idealization and planning the research, collecting data, and writing the manuscript. Josiane Ribolli: planning the research, analysis, and writing. Graziela Cleuza Vieira: implementation of experiments and collecting the data. João Paulo Ramos Ferreira: implementation of experiments and collecting the data. Carlos Henrique Araujo de Miranda Gomes: implementation of experiments and collecting the data. Claudio Manoel Rodrigues de Melo: funding acquisition, supervising the research activities, idealizing and planning the research, and revising the manuscript. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
According to Brazilian law, authorization for the use of invertebrates, including oysters, is not required in the conduct of scientific experiments.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Scaranto, B.M.S., Ribolli, J., Vieira, G.C. et al. Genetic Structure and Diversity in Wild and Cultivated Populations of the Mangrove Oyster Crassostrea gasar from Southern Brazil. Mar Biotechnol 25, 548–556 (2023). https://doi.org/10.1007/s10126-023-10224-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-023-10224-5