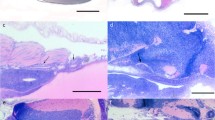
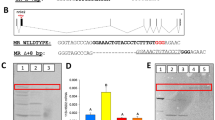
Abstract
The present study identified and characterized six key genes involved in the hypothalamic-pituitary-interrenal (HPI) axis of gilthead sea bream (Sparus aurata), a commercially important European aquaculture species. The key genes involved in the HPI axis for which gene structure and synteny analysis was carried out, comprised of two functional forms of glucocorticoid receptors (GR), as well as three forms of pro-opiomelanocortin (POMC) genes and one form of mineralocorticoid receptor (MR) gene. To explore their functional roles during development but also in the stress response, the expression profiles of gr1, gr2, mr, pomc_aI, pomc_aII, and pomc_β were examined during early ontogeny and after an acute stress challenge. The acute stress challenge was applied at the stage of full formation of all fins, where whole body cortisol was also measured. Both the cortisol and the molecular data implied that sea bream larvae at the stage of the full formation of all fins at 45 dph are capable of a response to stress of a similar profile as observed in adult fish.






Similar content being viewed by others
References
Acerete L, Balasch JC, Castellana B et al (2007) Cloning of the glucocorticoid receptor (GR) in gilthead seabream (Sparus aurata): differential expression of GR and immune genes in gilthead seabream after an immune challenge. Comp Biochem Physiol B Biochem Mol Biol 148:32–43
Alsop D, Vijayan M (2008) Development of the corticosteroid stress axis and receptor expression in zebrafish. Am J Phys Regul Integr Comp Phys 294:711–719
Alsop D, Vijayan MM (2009) Molecular programming of the corticosteroid stress axis during zebrafish development. Comp Biochem Physiol A Mol Integr Physiol 153:49–54
Barry TP, Malison JA, Held JA, Parish JJ (1995) Ontogeny of the cortisol stress response in larval rainbow trout. Gen Comp Endocrinol 97:57–65
Beato M, Chavez S, Truss M (1996) Transcriptional regulation by steroid hormones. Steroids 61:240–251
Bury NR, Sturm A (2007) Evolution of the corticosteroid receptor signaling pathway in fish. Gen Comp Endocrinol 153:47–56
Bury NR, Sturm A, Le Rouzic P, Lethimonier C, Ducouret B, Guiguen Y, Robinson-Rechavi M, Laudet V, Rafestin-Oblin ME, Prunet P (2003) Evidence for two distinct functional glucocorticoid receptors in teleost fish. J Mol Endocrinol 31:141–156
Cardoso JCR, Laiz-Carrion R, Louro B, Silva N, Canario AVM, Mancera JM, Power DM (2011) Divergence of duplicate POMC genes in gilthead sea bream Sparus auratus. Gen Comp Endocrinol 173:396–404
Cole TJ, Blendy JA, Monaghan AP, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schutz G (1995) Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev 9:1608–1621
De Jesus EGT, Hirano T (1992) Changes in whole body concentrations of cortisol, thyroid hormones, and sex steroids during early development of the chum salmon, Oncorhynchus keta. Gen Comp Endocrinol 85:55–61
De Jesus EG, Hirano T, Inui Y (1991) Changes in cortisol and thyroid hormone concentrations during early development and metamorphosis in Japanese flounder, Paralichthys olivaceus. Gen Comp Endocrinol 82:369–376
Dean DB, Whitlow ZW, Borski RJ (2003) Glucocorticoid receptor upregulation during seawater adaptation in a euryhaline teleost, the tilapia (Oreochromis mossambicus). Gen Comp Endocrinol 132:112–118
Deane EE, Woo NYS (2003) Ontogeny of thyroid hormones, cortisol, hsp70 and hsp90 during silver sea bream larval development. Life Sci 72:805–818
Di Bella ML, Vazzana M, Vizzini A, Parinello N (2008) Glucocorticoid receptor (DIGR1) is expressed in pre-larval and larval stages of the teleost fish Dicentrarchus labrax. Cell Tissue Res 333:39–47
Douglass J, Civelli O, Herbert E (1984) Polyprotein gene expression: generation of diversity of neuroendocrine peptides. Annu Rev Biochem 53:665–715
Evans RM (2005) The nuclear receptor superfamily: a rosetta stone for physiology. Mol Endocrinol 19:1429–1438
Fanouraki E, Mylonas CC, Papandroulakis N, Pavlidis M (2011) Species specificity in the magnitude and duration of the acute stress response in Mediterranean marine fish in culture. Gen Comp Endocrinol 173:313–322
Fuller PJ (1991) The steroid receptor superfamily: mechanisms of diversity. FASEB J 5:3092–3099
Greenwood AK, Butler PC, White RB, DeMarco U, Pearce D, Fernald RD (2003) Multiple corticosteroid receptors in a teleost fish: distinct sequences, expression patterns, and transcriptional activities. Endocrinology 144:4226–4236
Harris RM, Dijkstra PD, Hofmann HA (2014) Complex structural and regulatory evolution of the pro-opiomelanocortin gene family. Gen Comp Endocrinol 195:107–115
Hoegg S, Brinkmann H, Taylor JS, Meyer A (2004) Phylogenetic timing of the fish-specific genome duplication correlates with the diversification of teleost fish. J Mol Evol 59:190–203
Hu B, Jin J, Guo A-Y, Zhang H, Luo J, Gao G (2015) GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics 31:1296–1297
Hwang PP, Wu SM, Lin JH, Wu LS (1992) Cortisol content of eggs and larvae of teleosts. Gen Comp Endocrinol 86:189–196
Kiilerich P, Milla S, Sturm A, Valotaire C, Chevolleau S, Giton F, Terrien X, Fiet J, Fostier A, Debrauwer L, Prunet P (2011) Implication of the mineralocorticoid axis in rainbow trout osmoregulation during salinity acclimation. J Endocrinol 209:221–235
Kim BG, Brown CL (1997) Interaction of cortisol and thyroid hormone in the larval development of Pacific threadfin. Am Zool 37:470–481
Kumar R, Thompson EB (1999) The structure of the nuclear hormone receptors. Steroids 64:310–319
Kumar R, Byers RE, Munro AD, Lam TJ (1995) Profile of cortisol during ontogeny of the Asian seabass, Lates calcarifer. Aquaculture 132:349–359
Kumar R, Lee STL, Tan CH, Munro AD, Lam TJ (1997) Biosynthesis in vivo and excretion of cortisol by fish larvae. J Exp Zool 277:337–344
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Le Tallec PL, Lombes M (2005) The mineralocorticoid receptor: a journey exploring its diversity and specificity of action. Mol Endocrinol 19:2211–2221
Louis A, Muffato M, Roest Crollius H (2013) Genomicus: five genome browsers for comparative genomics in eukaryota. Nucleic Acids Res 41:D700–D705
Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M et al (1995) The nuclear receptor superfamily: the second decade. Cell 83:835–839
McCormick SD, Bradshaw D (2006) Hormonal control of salt and water balance in vertebrates. Gen Comp Endocrinol 147:3–8
McCormick SD, Regish A, O’Dea MF, Shrimpton JM (2008) Are we missing a mineralocorticoid in teleost fish? Effects of cortisol, deoxycorticosterone and aldosterone on osmoregulation, gill Na1, K1-ATPase activity and isoform mRNA levels in Atlantic salmon. Gen Comp Endocrinol 157:35–40
Metz JR, van den Burg EH, Bonga SE, Flik G (2003) Regulation of branchial Na(C)/K(C)-ATPase in common carp Cyprinus carpio L. acclimated to different temperatures. J Exp Biol 206:2273–2280
Milla S, Jalabert B, Rime H, Prunet P, Bobe J (2006) Hydration of rainbow trout oocyte during meiotic maturation and in vitro regulation by 17,20b-dihydroxy-4-pregnen-3-one and cortisol. J Exp Biol 209:1147–1156
Milla S, Terrien X, Sturm A, Ibrahim F, Giton F, Fiet J, Prunet P, le Gac F (2008) Plasma 11- deoxycorticosterone (DOC) and mineralocorticoid receptor testicular expression during rainbow trout Oncorhynchus mykiss spermiation: implication with 17alpha, 20beta-dihydroxyprogesterone on the milt fluidity? Reprod Biol Endocrinol 6:19
Mommsen T, Vijayan M, Moon T (1999) Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Rev Fish Biol Fish 9:211–268
Nelson JS (1994) Fishes of the world. Wiley and Sons, Hoboken
Nesan D, Kamkar M, Burrows J, Scott IC, Marsden M, Vijayan MM (2012) Glucocorticoid receptor signaling is essential for mesoderm formation and muscle development in zebrafish. Endocrinology 153:1288–1300
Pikulkaew S, Benato F, Celeghin A, Zucal C, Skobo T, Colombo L, Valle LD (2011) The knock-down of maternal glucocorticoid receptor mRNA alters embryo development in zebrafish. Dev Dyn 240:874–889
Prunet P, Sturm A, Milla S (2006) Multiple corticosteroid receptors in fish: from old ideas to new concepts. Gen Comp Endocrinol 147:17–23
Sakamoto T, Mori C, Minami S, Takahashi H, Abe T, Ojima D, Ogoshi M, Sakamoto H (2011) Corticosteroids stimulate the amphibious behavior in mudskipper: potential role of mineralocorticoid receptors in teleost fish. Physiol Behav 104:923–928
Sakamoto T, Yoshiki M, Takahashi H, Yoshida M, Ogino Y, Ikeuchi T, Nakamachi T, Konno N, Matsuda K, Sakamoto H (2016) Principal function of mineralocorticoid signaling suggested by constitutive knockout of the mineralocorticoid receptor in medaka fish. Sci Rep 6:37991
Sarropoulou E, Tsalafouta A, Sundaram AYM, Gilfillan GD, Kotoulas G, Papandroulakis N, Pavlidis M (2016) Transcriptomic changes in relation to early-life events in the gilthead sea bream (Sparus aurata). BMC Genomics 17:506–519
Slominski A, Wortsman J, Luger T, Paus R, Solomon S (2000) Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev 80:979–1020
Smith AI, Funder JW (1988) Proopiomelanocortin processing in the pituitary, central nervous system, and peripheral tissues. Endocr Rev 9:159–179
Stolte EH, Verburg van Kemenade BML, Savelkoul HFJ, Flik G (2006) Evolution of glucocorticoid receptors with different glucocorticoid sensitivity. J Endocrinol 190:17–28
Szisch V, Papandroulakis N, Fanouraki E, Pavlidis M (2005) Ontogeny of the thyroid hormones and cortisol in the gilthead sea bream, Sparus aurata. Gen Comp Endocrinol 142:186–192
Takahashi H, Sakamoto T (2012) The role of ‘mineralocorticoids’ in teleost fish: relative importance of glucocorticoid signaling in the osmoregulation and ‘central’ actions of mineralocorticoid receptor. Gen Comp Endocrinol 181:223–228
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10:512–526
Tsalafouta A, Papandroulakis N, Gorissen M, Katharios P, Flik G, Pavidis M (2014) Ontogenesis of the HPI axis and molecular regulation of the cortisol stress response during early development in Dicentrarchus labrax. Sci Rep 4:5525
Vandesompele J et al (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:RESEARCH0034
Vazzana M, Cammarata M, Parrinello N (2002) Confinement stress in sea bass (Dicentrarchus labrax) depresses peritoneal leukocyte cytotoxicity. Aquaculture 210:231–243
Vazzana M, Vizzini A, Sanfratello MA, Celi M, Salerno G, Parrinello N (2010) Differential expression of two glucocorticoid receptors in seabass (teleost fish) head kidney after exogeneous cortisol inoculation. Comp Biochem Physiol A 157:49–54
Volff JN (2005) Genome evolution and biodiversity in teleost fish. Heredity 94:280–294
Wendelaar Bonga SE (1997) The stress response in fish. Physiol Rev 77:591–625
Yeoh CG, Schreck CB, Feist GW, Fitzpatrick MS (1996) Endogenous steroid metabolism is indicated by fluctuations of endogenous steroid and steroid glucuronide levels in early development of the steelhead trout (Oncorhynchus mykiss). Gen Comp Endocrinol 103:107–114
Acknowledgements
We would like to thank Mr. N. Mitrizakis for his valuable assistance in larval rearing.
Funding
The research received funding from the European Union Seventh Framework Programme (FP7/2010-2014) under grant agreement no. [265957].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tsalafouta, A., Sarropoulou, E., Papandroulakis, N. et al. Characterization and Expression Dynamics of Key Genes Involved in the Gilthead Sea Bream (Sparus aurata) Cortisol Stress Response during Early Ontogeny. Mar Biotechnol 20, 611–622 (2018). https://doi.org/10.1007/s10126-018-9833-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-018-9833-5