Abstract
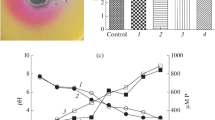
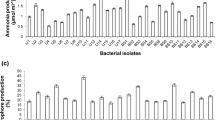
Salt affected cotton rhizospheric soil was explored for multi-stress resistance microbes to obtain 46 rhizobacteria. Of these, seven strains strongly inhibited the growth of phytopathogenic fungus Rhizoctonia solani by virtue of antifungal compound 2,4-diacetylphloroglucinol (DAPG) production. These seven strains demonstrated an array of plant growth-promoting activities as follows: (i) production of indole-3-acetic acid, ammonia, siderophore; (ii) solubilisation of phosphate, while two isolates showed Zn solubilisation. The phenetic and 16S ribotyping revealed affiliation of all the isolates to Pseudomonas guariconensis and presence of phlD gene marker for DAPG production. Among the seven isolates, strain VDA8 showed the highest DAPG production (0.16 μg ml−1) in liquid synthetic medium under aerobic conditions at 28 °C. Furthermore, sucrose, peptone, sodium hydrogen phosphate, ZnSO4, pH 8.0, and NaCl (1%) were observed as the best carbon, nitrogen, phosphate, trace element, pH, and salt concentration, respectively for maximum production of DAPG by strain VDA8 (3.62 ± 0.04 μg ml−1). The strain VDA8 was further assessed for wheat (Triticum aestivum) growth promotion by seed biopriming under laboratory (plate assay) and field condition in alkaline saline soil with pH 8.5. The field scale (324 m2) trials demonstrated 28.6% enhanced grain production compared to control demonstrating the newly isolated Pseudomonas sp. as multi-potent bioinoculant.






















Similar content being viewed by others
Data availability
The data that support the findings of this study available and sequence data was deposited to the GenBank under the accession numbers MN577429, MN577430, MN577431, MN577432, MN577433, MN577434, and MN577435.
Materials availability
The data supporting the findings of this study are available within the article and its supplementary materials.
References
Adebisi MA, Okelola FS, Alake CO, Ayo-Vaughan MA, Ajala MO (2010) Interrelationship between seed vigour traits and field performance in new rice for Africa (Nerica) genotypes (Oryza sativa L.). J Agric Sci Environ 10(2):15–24
Ali B, Sabri AN, Ljung K, Hasnain S (2009) Auxin production by plant associated bacteria: impact on endogenous IAA content and growth of Triticum aestivum L. Lett Appl Microbiol 48(5):542–547
Aliyas IM, Kassim GY, Mutlak NN (2015) Evaluation some germination characteristics for buckwheat seeds under experimental ecology conditions. Int J Sci Res Publ 5(11):634–638
Almario J, Bruto M, Vacheron J, Prigent-Combaret C, Moënne-Loccoz Y, Muller D (2017) Distribution of 2, 4-diacetylphloroglucinol biosynthetic genes among the Pseudomonas spp. reveals unexpected polyphyletism. Front Microbiol 8:1218
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410
Arora NK, Kang SC, Maheshwari DK (2001) Isolation of siderophore-producing strains of Rhizobium meliloti and their biocontrol potential against Macrophomina phaseolina that causes charcoal rot of groundnut. Curr Sci 81(6):673–677
Aydin MH (2022) Rhizoctonia solani and its biological control. Türkiye Tarımsal Araştırmalar Dergisi 9(1):118–135
Begum M, Rai VR, Lokesh S (2003) Effect of plant growth promoting rhizobacteria on seed borne fungal pathogens in okra. Indian phytopathol 56(2):156–158
Beneduzi A, Moreira F, Costa PB, Vargas LK, Lisboa BB, Favreto R, Baldani JI, Passaglia LM (2013) Diversity and plant growth promoting evaluation abilities of bacteria isolated from sugarcane cultivated in the South of Brazil. Appl Soil Ecol 63:94–104
Buysens S, Heungens K, Poppe J, Hofte M (1996) Involvement of pyochelin and pyoverdin in suppression of Pythium-induced damping-off of tomato by Pseudomonas aeruginosa 7NSK2. Appl Environ Microbiol 62(3):865–871
Chandra P, Wunnava A, Verma P, Chandra A, Sharma RK (2021) Strategies to mitigate the adverse effect of drought stress on crop plants—influences of soil bacteria: a review. Pedosphere 31(3):496–509
Chaubey S, Kotak M (2015) New method for isolation of plant probiotic fluorescent Pseudomonad and characterization for 2, 4-Diacetylphluoroglucinolproduction under different carbon sources and phosphate levels. Int J Plant Pathol Microbiol 6(2):1–7
Chen CY, Chang CK, Chang YW, Sue SC, Bai HI, Riang L, Hsiao CD, Huang TH (2007) Structure of the SARS coronavirus nucleocapsid protein RNA-binding dimerization domain suggests a mechanism for helical packaging of viral RNA. J Mol Biol 368(4):1075–1086
Combes-Meynet E, Pothier JF, Moënne-Loccoz Y, Prigent-Combaret C (2011) The Pseudomonas secondary metabolite 2, 4-diacetylphloroglucinol is a signal inducing rhizoplane expression of Azospirillum genes involved in plant-growth promotion. Mol Plant Microbe Interact 24(2):271–284
Daji JA, Kadam JR, Patil ND (1996) A text book of soil science. Media Promoters and Publishers Pvt. Ltd., Bombay
Dandi ND, Dandi BN, Chaudhari AB (2013) Bioprospecting of thermo-and osmo-tolerant fungi from mango pulp–peel compost for bioethanol production. Antonie Van Leeuwenhoek 103:723–736
Dane PR, Pawar SP, Kankariya RA, Chaudhari BL (2016) Pyoverdin mediated sunlight induced green synthesis of silver nanoparticles. RSC Adv 6(10):8503–8510
de Souza JT, Arnould C, Deulvot C, Lemanceau P, Gianinazzi-Pearson V, Raaijmakers JM (2003) Effect of 2, 4-diacetylphloroglucinol on Pythium: cellular responses and variation in sensitivity among propagules and species. Phytopathology 93(8):966–975
Devi PI, Thomas J, Raju RK (2017) Pesticide consumption in India: a spatiotemporal analysis §. Agric Econ Res Rev 30(1):163–172
Food and Agriculture Organization of The United Nations (2017) Averting risks to the food chain: a compendium of proven emergency prevention methods and tools. ISBN:978–92–5–109539–3
Freitas JR, Germida JJ (1990) Plant growth promoting rhizobacteria for winter wheat. Can J Microbiol 36(4):265–272
Gairola KC, Nautiyal AR, Dwivedi AK (2011) Effect of temperatures and germination media on seed germination of Jatropha curcas Linn. Advances in Bioresearch 2(2):66–71
Geetha K, Venkatesham E, Hindumathi A, Bhadraiah B (2014) Isolation, screening and characterization of plant growth promoting bacteria and their effect on Vigna Radita (L.) R. Wilczek. Int J Curr Microbiol Appl Sci 3(6):799–899
Ghevariya K, Desai P (2014) Rhizobacteria of sugarcane: in vitro screening for their plant growth promoting potentials. Res J Recent Sci ISSN 2277:2502
Goswami M, Suresh DE (2020) Plant growth-promoting rhizobacteria—alleviators of abiotic stresses in soil: a review. Pedosphere 30(1):40–61
Goswami D, Dhandhukia P, Patel P, Thakker JN (2014) Screening of PGPR from saline desert of Kutch: growth promotion in Arachis hypogea by Bacillus licheniformis A2. Microbiol Res 169(1):66–75
Haas D, Défago G (2005) Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol 3(4):307–319
Hontzeas N, Zoidakis J, Glick BR, Abu-Omar MM (2004) Expression and characterization of 1-aminocyclopropane-1-carboxylate deaminase from the rhizobacterium Pseudomonas putida UW4: a key enzyme in bacterial plant growth promotion. Biochim Biophys Acta (BBA)-Proteins Proteom 1703(1):11–19
Islam S, Akanda AM, Prova A, Islam MT, Hossain MM (2016) Isolation and identification of plant growth promoting rhizobacteria from cucumber rhizosphere and their effect on plant growth promotion and disease suppression. Front Microbiol 6:1360
Jousset A, Lara E, Wall LG, Valverde C (2006) Secondary metabolites help biocontrol strain Pseudomonas fluorescens CHA0 to escape protozoan grazing. Appl Environ Microbiol 72(11):7083–7090
Kankariya RA, Chaudhari AB, Gavit PM, Dandi ND (2019) 2, 4-Diacetylphloroglucinol: a novel biotech bioactive compound for agriculture. In: Singh D, Gupta V, Prabha R (eds) Microbial Interventions in Agriculture and Environment, Volume 1: Research Trends, Priorities and Prospects, Springer, Singapore, pp 419–452
Kankariya RA, Chaudhari AB, Dandi ND (2022a) Inhibitory efficacy of 2, 4-diacetylphloroglucinol against SARS-COV-2 proteins: in silico study. Biologia 77(3):815–828
Kankariya RA, Chaudhari AB, Dandi ND (2022b) Statistical production optimisation, Fusarium biocontrol and insecticidal activity of 2, 4-diacetylphloroglucinol produced by a newly discovered moderately haloalkalitolerant Pseudomonas guariconensis VDA8. Zemdirbyste-Agriculture 109(2):139–148
Kankariya RA, Chaudhari AB, Dandi ND (2023) Fusarium wilt biocontrol and plant growth promotion in Vigna radiata L. (Mung bean) by a drought resistant halo-alkali-tolerant Pseudomonas aeruginosa IRP169. Res J Agric Sci 14(2):445–455
Kavamura VN, Santos SN, da Silva JL, Parma MM, Ávila LA, Visconti A, Zucchi TD, Taketani RG, Andreote FD, de Melo IS (2013) Screening of Brazilian cacti rhizobacteria for plant growth promotion under drought. Microbiological research168(4):183–891
Kesaulya H, Zakaria B, Syaiful SA (2015) Isolation and physiological characterization of PGPR from potato plant rhizosphere in medium land of Buru Island. Procedia Food Science 3:190–199
Kobayashi T, Nishizawa NK (2012) Iron uptake, translocation, and regulation in higher plants. Annu Rev Plant Biol 63:131–152
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6):1547
Kumar A, Maurya BR, Raghuwanshi R (2021) The microbial consortium of indigenous rhizobacteria improving plant health, yield and nutrient content in wheat (Triticum aestivum). J Plant Nutr 44(13):1942–1956
Kwak YS, Weller DM (2013) Take-all of wheat and natural disease suppression: a review. The Plant Pathology Journal 29(2):125
Lisiecki K, Lemańczyk G, Piesik D, Mayhew CA (2022) Rhizoctonia cerealis and Rhizoctonia solani infection. Agriculture. Agriculture 12(12):1981
Lugojan C, Ciulca S (2011) Evaluation of relative water content in winter wheat. J Horticult Fore Biotechnol 15(2):173–177
Mahmood A, Turgay OC, Farooq M, Hayat R (2016) Seed biopriming with plant growth promoting rhizobacteria: a review. FEMS Microbiol Ecol 92(8):fiw112
Meyer SL, Everts KL, Gardener BM, Masler EP, Abdelnabby HM, Skantar AM (2017) Assessment of DAPG-producing for Management of and on Watermelon. J Nematol 48(1):43–53
Mirza MS, Mehnaz S, Normand P, Prigent-Combaret C, Moënne-Loccoz Y, Bally R, Malik KA (2006) Molecular characterization and PCR detection of a nitrogen-fixing Pseudomonas strain promoting rice growth. Biol Fertil Soils 43:163–170
Moeinzadeh A, Sharif-Zadeh F, Ahmadzadeh M, Tajabadi FH (2010) Biopriming of sunflower (‘Helianthus annuus’ L.) seed with ‘Pseudomonas fluorescens’ for improvement of seed invigoration and seedling growth. Australian J Crop Sci 4(7):564–570
Moshynets OV, Babenko LM, Rogalsky SP, Iungin OS, Foster J, Kosakivska IV, Potters G, Spiers AJ (2019) Priming winter wheat seeds with the bacterial quorum sensing signal N-hexanoyl-L-homoserine lactone (C6-HSL) shows potential to improve plant growth and seed yield. PLoS ONE 14(2):e0209460
Mut Z, Akay H (2010) Effect of seed size and drought stress on germination and seedling growth of naked oat (Avena sativa L.). Bulg J Agric Sci 16(4):459–467
Nagel K, Schneemann I, Kajahn I, Labes A, Wiese J, Imhoff JF (2012) Beneficial effects of 2, 4-diacetylphloroglucinol-producing pseudomonads on the marine alga Saccharina latissima. Aquat Microb Ecol 67(3):239–249
Ownley BH, Duffy BK, Weller DM (2003) Identification and manipulation of soil properties to improve the biological control performance of phenazine-producing Pseudomonas fluorescens. Appl Environ Microbiol 69(6):3333–3343
Patten CL, Glick BR (2002) Regulation of indoleacetic acid production in Pseudomonas putida GR12-2 by tryptophan and the stationary-phase sigma factor RpoS. Can J Microbiol 48(7):635–642
Pradhan N, Sukla LB (2006) Solubilization of inorganic phosphates by fungi isolated from agriculture soil. Afr J Biotechnol 5(10)
Quecine MC, Kidarsa TA, Goebel NC, Shaffer BT, Henkels MD, Zabriskie TM, Loper JE (2016) An interspecies signaling system mediated by fusaric acid has parallel effects on antifungal metabolite production by Pseudomonas protegens strain Pf-5 and antibiosis of Fusarium spp. Appl Environ Microbiol 82(5):1372–1382
Raaijmakers JM, Weller DM, Thomashow LS (1997) Frequency of antibiotic-producing Pseudomonas spp. in natural environments. Appl Environ Microbiol 63(3):881–887
Reddy KR, Choudary KA, Reddy MS (2007) Antifungal metabolites of Pseudomonas fluorescens isolated from rhizosphere of rice crop. J Mycol Pl Pathol 37(2):1–5
Rehman A, Farooq M, Naveed M, Nawaz A, Shahzad B (2018) Seed priming of Zn with endophytic bacteria improves the productivity and grain biofortification of bread wheat. Eur J Agron 94:98–107
Rovera M, Cabrera S, Rosas S, Correa N (2002) Characterization of antifungal agents produced by Pseudomonas aurantiaca for its use as biocontrol inoculant. In: Pedrosa FO, Hungria M, Yates G, Newton WE (eds) Nitrogen Fixation: From Molecules to Crop Productivity. Current Plant Science and Biotechnology in Agriculture vol 38. Springer Dordrecht. https://doi.org/10.1007/0-306-47615-0_343
Ruan S, Xue Q, Tylkowska K (2002) The influence of priming on germination of rice (Oryza sativa L.) seeds and seedling emergence and performance in flooded soil. Seed Sci Technol 30(1):61–67
Safari D, Jamali F, Nooryazdan HR, Bayat F (2016) Screening fluorescent pseudomonads isolated from wheat rhizosphere for plant growth-promoting and salt tolerance properties. Biol Forum– Int J 8(1):35–42
Saharan K, Sarma MV, Prakash A, Johri BN, Bisaria VS, Sahai V (2011) Shelf-life enhancement of bio-inoculant formulation by optimizing the trace metals ions in the culture medium for production of DAPG using fluorescent pseudomonad R62. Enzyme Microb Technol 48(1):33–38
Shahi C, Vibhuti KB, Bargali SS (2015) How seed size and water stress affect the seed germination and seedling growth in wheat varieties. Curr Agric Res J 3(1):60–68
Shanthi AT, Vittal RR (2013) Biocontrol potentials of plant growth promoting rhizobacteria against Fusarium wilt disease of cucurbit. International Journal of Phytopathology 2(3):155–161
Sharifi RS (2012) Study of nitrogen rates effects and seed biopriming with PGPR on quantitative and qualitative yield of safflower (Carthamus tinctorius L.). Technical J Eng Appl Sci 2(7):162–166
Sharma S, Kooner R, Arora R (2017) Insect Pests and Crop Losses. In: Arora R, Sandhu S (eds) Breeding Insect Resistant Crops for Sustainable Agriculture. Springer, Singapore. https://doi.org/10.1007/978-981-10-6056-4_2
Shirzad A, Fallahzadeh-Mamaghani V, Pazhouhandeh M (2012) Antagonistic potential of fluorescent pseudomonads and control of crown and root rot of cucumber caused by Phythophtora drechsleri. Plant Pathol J 28(1):1–9
Sid’Ko AF, Botvich IY, Pisman TI, Shevyrnogov AP (2017) Estimation of chlorophyll content and yield of wheat crops from reflectance spectra obtained by ground-based remote measurements. Field Crop Res 207:24–29
Soltani E, Galeshi S, Kamkar B, Akramghaderi F (2008) Modeling seed aging effects on the response of germination to temperature in wheat. Seed Sci Biotechnol 2(1):32–36
Somwanshi RB, Kadu PP, Tamboli BD, Patil YM, Bhakare BD (1969) Analysis of plants, Irrigation water & soils. In: Determination of soil salinity and Elecrical conductivity‟ –saturated paste method, Richard 284:229–235
Steiner F, Zuffo AM, Zoz T, Zoz A, Zoz J (2017) Drought tolerance of wheat and black oat crops at early stages of seedling growth. Revista de Ciências Agrárias 40(3):576–586
Steiner F, Zuffo AM, Busch A, Sousa TD, Zoz T (2019) Does seed size affect the germination rate and seedling growth of peanut under salinity and water stress? Pesquisa Agropecuária Tropical 49
Tada M, Takakuwa T, Nagai M, Yoshii T (1990) Antiviral and antimicrobial activity of 2, 4-diacylphloroglucinols, 2-acylcyclohexane-1, 3-diones and 2-carboxamidocyclo-hexane-1, 3-diones. Agric Biol Chem 54(11):3061–3063
Tamura K, Stecher G, Kumar S (2021) MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38(7):3022–3027
Tang A, Haruna AO, Majid NM, Jalloh MB (2020) Potential PGPR properties of cellulolytic, nitrogen-fixing, phosphate-solubilizing bacteria in rehabilitated tropical forest soil. Microorganisms 8(3):442
Terada H (1981) The interaction of highly active uncouplers with mitochondria. Biochim Biophys Acta (BBA)-Rev Bioener 639(34):225–242
Timmusk S, Behers L, Muthoni J, Muraya A, Aronsson AC (2017) Perspectives and challenges of microbial application for crop improvement. Front Plant Sci 8:49
Troppens DM, Chu M, Holcombe LJ, Gleeson O, O’Gara F, Read ND, Morrissey JP (2013) The bacterial secondary metabolite 2, 4-diacetylphloroglucinol impairs mitochondrial function and affects calcium homeostasis in Neurospora crassa. Fungal Genet Biol 56:135–146
Turan M, Gulluce M, Şahin F (2012) Effects of plant-growth-promoting rhizobacteria on yield, growth, and some physiological characteristics of wheat and barley plants. Commun Soil Sci Plant Anal 43(12):1658–1673
Veena VK, Kennedy K, Lakshmi P, Krishna R, Sakthivel N (2016) Anti-leukemic, anti-lung, and anti-breast cancer potential of the microbial polyketide 2, 4-diacetylphloroglucinol (DAPG) and its interaction with the metastatic proteins than the antiapoptotic Bcl-2 proteins. Mol Cell Biochem 414:47–56
Velusamy P, Immanuel JE, Gnanamanickam SS (2013) Rhizosphere bacteria for biocontrol of bacterial blight and growth promotion of rice. Rice Sci 20(5):356–362
Weller DM, Mavrodi DV, van Pelt JA, Pieterse CM, van Loon LC, Bakker PA (2012) Induced systemic resistance in Arabidopsis thaliana against Pseudomonas syringae pv. tomato by 2, 4-diacetylphloroglucinol-producing Pseudomonas fluorescens. Phytopathology 102(4):403–412
Yin C, Hulbert SH, Schroeder KL, Mavrodi O, Mavrodi D, Dhingra A, Schillinger WF, Paulitz TC (2013) Role of bacterial communities in the natural suppression of Rhizoctonia solani bare patch disease of wheat (Triticum aestivum L.). Appl Environ Microbiol 79(23):7428–7438
Yuan BC, Li ZZ, Liu H, Gao M, Zhang YY (2007) Microbial biomass and activity in salt affected soils under arid conditions. Appl Soil Ecol 35(2):319–328
Zhang W, Zhao Z, Zhang B, Wu XG, Ren ZG, Zhang LQ (2014) Posttranscriptional regulation of 2, 4-diacetylphloroglucinol production by GidA and TrmE in Pseudomonas fluorescens 2P24. Appl Environ Microbiol 80(13):3972–3981
Zhang J, Han W, Huang L, Zhang Z, Ma Y, Hu Y (2016) Leaf chlorophyll content estimation of winter wheat based on visible and near-infrared sensors. Sensors 16(4):437
Zhou T, Chen D, Li C, Sun Q, Li L, Liu F, Shen Q, Shen B (2012) Isolation and characterization of Pseudomonas brassicacearum J12 as an antagonist against Ralstonia solanacearum and identification of its antimicrobial components. Microbiol Res 167(7):388–394
Zhou L, Jiang HX, Sun S, Yang DD, Jin KM, Zhang W, He YW (2016) Biotechnological potential of a rhizosphere Pseudomonas aeruginosa strain producing phenazine-1-carboxylic acid and phenazine-1-carboxamide. World J Microbiol Biotechnol 32:1–2
Funding
The work is supported by the Department of Science and Technology (DST, New Delhi India) WOS-A (DST letter no. SR/WOS-A/LS-1209/2014(G) dated 12.05.2015) and Dr. Navin D. Dandi as a mentor. We also acknowledge Rajiv Gandhi Science and Technology Commission, Government of Maharashtra (KBCNMU/RGSTC/Sanction Order/30 dated 25.02.2020), University Grants Commission (U.G.C), New Delhi, and Department of Science and Technology (D.S.T), New Delhi, for funding under SAP and FIST program respectively, to the university. The authors are also thankful to JITC Park, Jalgaon, for field trial support.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Kankariya Raksha A., Jape Prasad V., Patil Rajkamal P., Chaudhari Ambalal B., and Dandi Navin D. The first draft of the manuscript was written by Kankariya Raksha A. and finally, authors Chaudhari Ambalal B. and Dandi Navin D. read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kankariya, R.A., Jape, P.V., Patil, R.P. et al. Bioprospecting of multi-stress tolerant Pseudomonas sp. antagonistic to Rhizoctonia solani for enhanced wheat growth promotion. Int Microbiol (2024). https://doi.org/10.1007/s10123-024-00517-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10123-024-00517-7