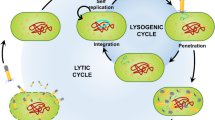
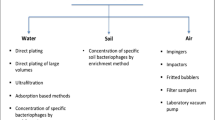
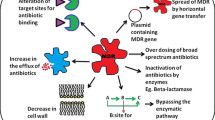
Abstract
Although bacteriophages (or simply phages) are the most abundant biological entities and have the potential to transfer genetic material between bacterial hosts, their contribution to the acquisition and spread of antibiotic resistance genes in the environment has not been extensively studied. The environment is continually exposed to a wide variety of pollutants from anthropogenic sources, which may promote horizontal gene transfer events, including those mediated by phages. Considering the significant and growing concern of antibiotic resistance, phages should be taken into consideration during the implementation of mitigation measures. This review is focused on the emergence and spread of antibiotic resistance in the environment, with a special emphasis on the role of phages.

Similar content being viewed by others
References
Abeles SR, Ly M, Santiago-Rodriguez TM, Pride DT (2015) Effects of long term antibiotic therapy on human oral and fecal viromes. PLoS One 10:e0134941
Anand T, Bera BC, Vaid RK, Barua S, Riyesh T, Virmani N, Hussain M, Singh RK, Tripathi BN (2016) Abundance of antibiotic resistance genes in environmental bacteriophages. J Gen Virol 97:3458–3466
Caflisch KM, Patel R (2019) Implications of bacteriophage- and bacteriophage component-based therapies for the clinical microbiology laboratory. J Clin Microbiol 57:e00229–e00219
Calero-Cáceres W, Balcázar JL (2019) Antibiotic resistance genes in bacteriophages from diverse marine habitats. Sci Total Environ 654:452–455
Calero-Cáceres W, Muniesa M (2016) Persistence of naturally occurring antibiotic resistance genes in the bacteria and bacteriophage fractions of wastewater. Water Res 95:11–18
Calero-Cáceres W, Ye M, Balcázar JL (2019) Bacteriophages as environmental reservoirs of antibiotic resistance. Trends Microbiol 27:570–777
Callanan J, Stockdale SR, Shkoporov A, Draper LA, Ross RP, Hill C (2018) RNA phage biology in a metagenomic era. Viruses 10:386
Chen J, Quiles-Puchalt N, Chiang YN, Bacigalupe R, Fillol-Salom A, Chee MSJ, Fitzgerald JR, Penadés JR (2018) Genome hypermobility by lateral transduction. Science 362:207–212
Colomer-Lluch M, Jofre J, Muniesa M (2011) Antibiotic resistance genes in the bacteriophage DNA fraction of environmental samples. PLoS One 6:e17549
Debroas D, Siguret C (2019) Viruses as key reservoirs of antibiotic resistance genes in the environment. ISME J 13:2856–2867
Enault F, Briet A, Bouteille L, Roux S, Sullivan MB, Petit MA (2017) Phages rarely encode antibiotic resistance genes: a cautionary tale for virome analyses. ISME J 11:237–247
Gandra S, Barter DM, Laxminarayan R (2014) Economic burden of antibiotic resistance: how much do we really know? Clin Microbiol Infect 20:973–980
Hatfull GF (2015) Dark matter of the biosphere: the amazing world of bacteriophage diversity. J Virol 89:8107–8110
Hilbert M, Csadek I, Auer U, Hilbert F (2017) Antimicrobial resistance-transducing bacteriophages isolated from surfaces of equine surgery clinics-a pilot study. Eur J Microbiol Immunol 7:296–302
Jiang SC, Paul JH (1998) Gene transfer by transduction in the marine environment. Appl Environ Microbiol 64:2780–2787
Kenzaka T, Tani K, Nasu M (2010) High-frequency phage-mediated gene transfer in freshwater environments determined at single-cell level. ISME J 4:648–659
Koskella B, Meaden S (2013) Understanding bacteriophage specificity in natural microbial communities. Viruses 5:806–823
Lekunberri I, Subirats J, Borrego CM, Balcázar JL (2017a) Exploring the contribution of bacteriophages to antibiotic resistance. Environ Pollut 220:981–984
Lekunberri I, Villagrasa M, Balcázar JL, Borrego CM (2017b) Contribution of bacteriophage and plasmid DNA to the mobilization of antibiotic resistance genes in a river receiving treated wastewater discharges. Sci Total Environ 601–602:206–209
Livermore DM, Blaser M, Carrs O, Cassell G, Fishman N, Guidos R, Levy S, Powers J, Norrby R, Tillotson G, Davies R, Projan S, Dawson A, Monnet D, Keogh-Brown M, Hand K, Garner S, Findlay D, Morel C, Wise R, Bax R, Burke F, Chopra I, Czaplewski L, Finch R, Piddock LJV, White T (2011) Discovery research: the scientific challenge of finding new antibiotics. J Antimicrob Chemother 66:1941–1944
Marti E, Variatza E, Balcázar JL (2014a) The role of aquatic ecosystems as reservoirs of antibiotic resistance. Trends Microbiol 22:36–41
Marti E, Variatza E, Balcázar JL (2014b) Bacteriophages as a reservoir of extended-spectrum β-lactamase and fluoroquinolone resistance genes in the environment. Clin Microbiol Infect 20:O456–O459
Modi SR, Lee HH, Spina CS, Collins JJ (2013) Antibiotic treatment expands the resistance reservoir and ecological network of the phage metagenome. Nature 499:219–222
Novick R (1967) Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology 33:155–166
Rizzo L, Manaia C, Merlin C, Schwartz T, Dagot C, Ploy MC, Michael I, Fatta-Kassinos D (2013) Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: a review. Sci Total Environ 447:345–360
Rolain JM, Abat C, Jimeno MT, Fournier PE, Raoult D (2016) Do we need new antibiotics? Clin Microbiol Infect 22:408–415
Roshini J, Raj M, Karunasagar I (2017) Prevalence of blaCTX-M-15 in coliphages isolated from sewage. Adv Sci Lett 23:1869–1871
Ross J, Topp E (2015) Abundance of antibiotic resistance genes in bacteriophage following soil fertilization with dairy manure or municipal biosolids, and evidence for potential transduction. Appl Environ Microbiol 81:7905–7913
Shousha A, Awaiwanont N, Sofka D, Smulders FJ, Paulsen P, Szostak MP, Humphrey T, Hilbert F (2015) Bacteriophages isolated from chicken meat and the horizontal transfer of antimicrobial resistance genes. Appl Environ Microbiol 81:4600–4606
Shrestha P, Cooper BS, Coast J, Oppong R, Do Thi Thuy N, Phodha T, Celhay O, Guerin PJ, Wertheim H, Lubell Y (2018) Enumerating the economic cost of antimicrobial resistance per antibiotic consumed to inform the evaluation of interventions affecting their use. Antimicrob Resist Infect Control 7:98
Singer AC, Shaw H, Rhodes V, Hart A (2016) Review of antimicrobial resistance in the environment and its relevance to environmental regulators. Front Microbiol 7:1728
Smith HW (1972) Ampicillin resistance in Escherichia coli by phage infection. Nat New Biol 238:205–206
Torres-Barceló C (2018) The disparate effects of bacteriophages on antibiotic-resistant bacteria. Emerg Microbes Infect 7:168
Wang M, Xiong W, Liu P, Xie X, Zeng J, Sun Y, Zeng Z (2018a) Metagenomic insights into the contribution of phages to antibiotic resistance in water samples related to swine feedlot wastewater treatment. Front Microbiol 9:2474
Wang M, Liu P, Zhou Q, Tao W, Sun Y, Zeng Z (2018b) Estimating the contribution of bacteriophage to the dissemination of antibiotic resistance genes in pig feces. Environ Pollut 238:291–298
Ye M, Sun M, Huang D, Zhang Z, Zhang H, Zhang S, Hu F, Jiang X, Jiao W (2019) A review of bacteriophage therapy for pathogenic bacteria inactivation in the soil environment. Environ Int 129:488–496
Zhang A, Call DR, Besser TE, Liu J, Jones L, Wang H, Davis MA (2019) β-lactam resistance genes in bacteriophage and bacterial DNA from wastewater, river water, and irrigation water in Washington State. Water Res 161:335–340
Zinder ND, Lederberg J (1952) Genetic exchange in Salmonella. J Bacteriol 64:679–699
Acknowledgments
I thank the Spanish Society for Microbiology (SEM) for honoring me with the Jaime Ferran Award 2019.
Funding
This work was supported by the Economy and Knowledge Department of the Catalan Government through Consolidated Research Group (ICRA-ENV 2017 SGR 1124).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Balcázar, J.L. Implications of bacteriophages on the acquisition and spread of antibiotic resistance in the environment. Int Microbiol 23, 475–479 (2020). https://doi.org/10.1007/s10123-020-00121-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10123-020-00121-5