Abstract
Background
Recent studies showed inverse relationship between hypercholesterolemia and the risk of gastric cancer, especially among male. However evidence among female is inconsistent. We aimed to investigate the relationship between cholesterol level and the risk of gastric cancer among female according to menopausal status.
Methods
We analyzed the data from a population-based prospective cohort of female ≥ 30 years old who underwent cancer screening and general health screening provided by the Korean National Health Insurance Corporation in 2009. Under quartile stratification of the level of cholesterol components, we calculated the hazard ratio (HR) for gastric cancer incidence until 2018 for each level group according to the menopausal status at 2009.
Results
Among total 2,722,614 individuals, 17,649 gastric cancer cases developed after mean 8.26 years of follow-up (premenopausal 3746/1180666; postmenopausal 13,903/1541948). Total cholesterol, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) showed inverse relationship with the risk of gastric cancer among postmenopausal women (adjusted HR (95% confidence interval) for the highest quartile vs. lowest quartile and p-for-trend: 0.88 (0.84–0.92) and < 0.001 for total cholesterol; 0.89 (0.85–0.92) and < 0.001 for HDL-C; 0.92 (0.89–0.97) and 0.001 for LDL-C), whereas none showed statistically significant risk relationship among premenopausal women. Triglyceride was not independently related with gastric cancer risk among both pre- and postmenopausal women.
Conclusions
Cholesterol levels, including total cholesterol, HDL-C, and LDL-C, are inversely related with the risk of gastric cancer among postmenopausal women, but not among premenopausal women.
Similar content being viewed by others
Introduction
Hypercholesterolemia has been known as a major cause of cardiovascular diseases [1, 2]. On the other hand, the relationship between cholesterol level and risk of cancer is still uncertain. Several cancers, such as prostate [3, 4], breast [4], and rectal cancer [5], have a positive correlation with hyperlipidemia. In contrast, in a number of prospective studies, total cholesterol level was shown to be inversely associated with risk for cancer [3, 6,7,8,9,10,11], although this might be explained by reverse causation [12].
Regarding gastric cancer, several studies have shown an inverse association between cholesterol level and gastric cancer risk [3, 4, 13, 14]. However, in most of the studies, the inverse relationship was prominent among men, whereas the evidence was inconsistent among women [3, 4, 13]. The reason for such difference might be because of sex hormone, as cholesterol is closely related with sex hormone as its major precursor molecule [15].
Gastric cancer is known to have high male predominance, and the male-to-female ratio is relatively consistent worldwide in various populations with different gastric cancer incidences and unchanged over decades [16]. This is usually attributed to the protective effect of female sex hormone against gastric carcinogenesis [16,17,18], although the underlying mechanism is not well known yet. So far, it is thought to be the anti-inflammatory [19,20,21] and antioxidant effect exerted by estrogen receptors [22].
We assumed that the effect of cholesterol on gastric cancer risk might be affected by female sex hormone. In this study, we aimed to investigate the relationship between cholesterol and gastric cancer incidence according to menopausal status among female using a large-scale population-based prospective cohort in the area of high gastric cancer incidence.
Methods
Study population
We used a prospectively collected population-based female cohort which underwent both cancer screening and general health screening provided by the Korean National Health Insurance Corporation (NHIC) in 2009. The NHIC covers up to 97% of Korean population and offers standardized general health screening for all individuals ≥ 20 years old and employees of any age at least biennially and supplies national cancer screening for major 5 cancers: colorectal (≥ 50 years old), liver (high-risk individuals ≥ 40 years old), gastric (≥ 40 years old), breast (females ≥ 40 years old), and cervical cancer (females ≥ 20 years old).
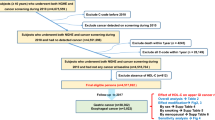
As the starting age for cervical cancer screening has been 30 until 2016, in this study we enrolled female subjects ≥ 30 years old at the time of screening in 2009. Among the total 3,280,834 female subjects who had both national cancer screening and general health screening in 2009, those who had hysterectomy (n = 206,481), those diagnosed with any type of cancer before the enrollment (n = 64,036) were excluded. Those who had previous hysterectomy were excluded because their hormonal menopausal status is unclear due to the surgical menopause. Also, those with missing data about lipid profiles or menopausal status (n = 257,487) and those who developed gastric cancer within one year after the enrollment (n = 30,216) were excluded. The last were excluded to minimize reverse causality between lipid and gastric cancer as there are possibilities that those with undiagnosed gastric cancer may induce hypocholesterolemia due to its catabolic effect. Thus the remaining 2,722,614 individuals were finally included in this study (Fig. 1). Follow-up duration was calculated from the general health screening date until the new diagnosis of gastric cancer, death, or the end of the study date of December 31st, 2018, whichever came first.
The Institutional Review Board of the Seoul National University Bundang Hospital approved this study (IRB No.X-1608/360-906). This study complied with the Declaration of Helsinki in 1964. Informed consent was waived because of the retrospective design; however, this study excluded any personal identification information and the Institutional Review Board approved the waiver of informed consent. All authors had access to the study data and reviewed and approved the final manuscript.
Clinical parameters and biochemical analysis
Information about menopausal status and health behaviors was achieved from the standardized self-reporting questionnaire completed on the day of screening before the examinations. In the questionnaire, following data were extracted: previous cancer diagnosis, menopausal status, previous hysterectomy, history of hormone replacement therapy (HRT), smoking (never/former/current), alcohol (none/ < 30 g/day/ ≥ 30 g/day), and regular exercise (high-intensity for > 20 min ≥ 3 times/week or moderate-intensity for > 30 min ≥ 5 times/week over the last week, yes/no).
Anthropometric data including height (m), weight (kg), waist circumference (cm), systolic/diastolic blood pressure (mmHg) were examined on the day of screening.
Laboratory data including fasting serum glucose (mg/dL), cholesterol (total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglyceride (TG)) (mg/dL), and glomerular filtration rate (GFR) (mL/min/m2) were measured after ≥ 8 h of fasting.
Definitions
Gastric cancer diagnosis was identified from the claim data using the International Classification of Diseases, 10th revision (ICD-10) code of C16.
Diabetes was defined as a fasting plasma glucose ≥ 126 mg/dL, taking oral hypoglycemic agents/insulin, or having the ICD-10 code of E11-14. Hypertension was defined as systolic/diastolic blood pressure ≥ 140/90 mmHg; taking antihypertensive medication; or having the ICD-10 code, I10-13. Dyslipidemia was defined as fasting total cholesterol ≥ 240 mg/dL, taking lipid-lowering medication, or having the ICD-10 code E78. Any medication history was defined as history of prescription within one year before the enrollment by the claim data.
Statistical analysis
To analyze continuous and categorical variables, Student’s t test and Chi-square test were used, respectively.
Gastric cancer incidence rates were calculated as the numbers of cases per 1000 person-years at risk. To investigate the independent risk of lipid profiles on gastric cancer development, Cox proportional hazard model was used under adjustment for clinically important factors. For time-to-event analyses, log-rank test was used.
All the analyses were performed by using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R version 3.2.3 (The R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org). A p value < 0.05 was considered statistically significant and all tests were two sided.
Results
Baseline characteristics
The baseline characteristics of the study population according to the menopausal status are shown in the Table 1. Among the total, 1,521,948 were menopausal at 2009 and 1,180,666 were not. Postmenopausal women were older, with higher blood pressure, BMI, waist circumference, cholesterol, fasting serum glucose, and with lower GFR compared with premenopausal women (all p < 0.001). Also postmenopausal women were less likely to smoke or drink alcohol, more likely to exercise regularly, and more commonly have comorbidity like diabetes, hypertension, or dyslipidemia compared with premenopausal women (all p < 0.001).
Lipid and gastric cancer risk among total
In this population, gastric cancer developed in 17,649 subjects (0.65%), including 3746 premenopausal women (0.31%) and 13,903 postmenopausal women (0.90%).
To investigate the relationship between lipids and gastric cancer incidence, hazard ratios (HRs) for gastric cancer in each quartile of lipid components and p-for-trend were calculated. Without any adjustment (model-1), total cholesterol, LDL-C, TG were shown to be positively related with gastric cancer, while HDL-C showed inverse relationship (Table 2). These associations were all statistically significant. However, under age adjustment (model-2), the correlation of LDL-C and TG with gastric cancer disappeared (p-for-trend: 0.19 for LDL-C; 0.10 for TG), and that of total cholesterol moved to inverse (p-for-trend = 0.01). Meanwhile, the inverse relationship between HDL-C and gastric cancer incidence remained significant after age adjustment (p-for-trend < 0.001). When we further adjusted them for multiple clinically important factors, including age, smoking, alcohol, regular exercise, BMI, diabetes, and lipid-lowering agents (model-3), LDL-C and TG did not show any independent association with gastric cancer, whereas total cholesterol and HDL-C consistently revealed a significant inverse relationship with gastric cancer.
Lipid and gastric cancer risk according to menopausal status
In premenopausal women, total cholesterol, LDL-C, and TG were shown to be positively related with gastric cancer under no adjustment (model-1), as in the total (p-for-trend: < 0.001 for total cholesterol; < 0.001 for LDL-C; < 0.001 for TG) (Table 2), while HDL-C showed inverse trend although each quartile did not show statistically significant result (p-for-trend = 0.02). However, such relationships all disappeared after adjustment with age (model-2), or multiple factors (model-3).
Postmenopausal women showed totally different trends from those in premenopausal women. In postmenopausal women, under no adjustment (model-1), total cholesterol, HDL-C, and LDL-C revealed inverse relationship with gastric cancer (all p-for-trend < 0.001), while TG showed positive relationship (p-for-trend < 0.001), with dose–response manner. The inverse relationship of total cholesterol, HDL-C, and LDL-C remained (p-for-trend: < 0.001 for total cholesterol and HDL-C; 0.01 for LDL-C), whereas that of TG disappeared (p-for-trend = 0.27) after age adjustment (model-2). Such inverse association of total cholesterol, HDL-C, and LDL-C remained significant after adjustment for multiple factors (model-3) (p-for-trend: < 0.001 for total cholesterol and HDL-C; 0.001 for LDL-C).
Subgroup analysis among postmenopausal women according to HRT
To minimize the potential bias by artificially replaced female sex hormone, we further performed subgroup analysis among postmenopausal women according to HRT. Table 3 shows the HRs and p-for-trend under no adjustment (model-1), age adjustment (model-2), and adjustment for multiple factors (model-3).
Among those without HRT, the effect trends of each lipid components for the gastric cancer risk were almost the same with those among the overall postmenopausal women, showing risk-lowering trends for total cholesterol, HDL-C, and LDL-C, while null relationship for TG. However, the risk-lowering effects of total cholesterol and LDL-C became prominent and the statistical significance for the effect of LDL-C was increased after exclusion of those with HRT.
Whereas, among those with HRT, almost none of the quartiles of each lipid components showed statistically significant association with gastric cancer risk, except for HDL-C. HDL-C showed inverse relationship with gastric cancer incidence under no adjustment (model-1) (p-for-trend < 0.001), age adjustment (model-2) (p-for-trend < 0.001), and even under multi-factor adjustment (model-3) (p-for-trend = 0.001). Although TG showed positive association under no adjustment (model-1) (p-for-trend = 0.03), it failed to show statistically significant relationship after adjustment with age (model-2) (p-for-trend = 0.71) or multiple factors (model-3) (p-for-trend = 0.92).
Time-to-event analyses for gastric cancer incidence
We also performed log-rank tests for gastric cancer incidence according to menopausal status. To minimize potential bias, those analyses were performed under age stratification. Pre- and postmenopausal women were subdivided into two groups with cut-off age of 45 and 55, respectively. Among premenopausal women, every lipid component failed to show any statistical relationship with gastric cancer development regardless with age, only except for LDL-C among those < 45 years old, which showed p value of 0.04; however, the Kaplan–Meier curve showed no consistent trend among the quartiles (Supplementary Fig. 1). Among postmenopausal women, HDL-C consistently showed statistically significant trend of inverse relationship with gastric cancer incidence in both age subgroups (p: < 0.01 for age < 55; < 0.0001 for age ≥ 55) (Supplementary Fig. 2). Total cholesterol and LDL-C showed statistically significant inverse relationship with gastric cancer only among those ≥ 55 years old (both p < 0.0001), while they did not among those < 55 years old (p: 0.63 for total cholesterol; 0.84 for LDL-C). TG showed positive relationship with gastric cancer among those ≥ 55 years old (p < 0.0001), while it did not among those < 55 years old (p = 0.75).
Gastric cancer incidence according to statin medication
To evaluate the effect of statin medication, gastric cancer incidence according to the presence or absence of statin medication was analyzed. The relationship between statin medication and gastric cancer risk was shown to be similar to that between hypercholesterolemia and gastric cancer. That is, women taking statin showed lower risk of gastric cancer in the entire cohort. Compared with those without statin medication, those taking statin had lower risk of gastric cancer among postmenopausal women. However, this was not significant among premenopausal women after adjustment with covariates (Supplementary Table 1).
Discussion
This study indicates that the higher level of total cholesterol, HDL-C, and LDL-C are related with the lower risk of gastric cancer in postmenopausal women, especially women over 55 years of age, but not in premenopausal women. The highest quartile of the three lipid components decreased gastric cancer risk by approximately 10% and also dose–response relationship was approved. Such inverse relationship between lipid and gastric cancer incidence was true only for HDL-C among postmenopausal women under HRT.
This result shows that the relationship between serum lipid and gastric cancer risk is largely affected by menopausal status. For this phenomenon, two hypotheses can be conceived. First, the different results between pre- and postmenopausal women are due to the female hormonal status. Second, the difference is because of the different age or age-related environmental factors. However, considering the previous researches demonstrating generally consistent results of inverse relationship between cholesterol and gastric cancer incidence among men and inconsistent results among women [3, 4, 13], the former hypothesis seems more likely true. Also, the fact that subgroup analysis among postmenopausal women under HRT failed to show the inverse relationship or attenuated the significance further support the former hypothesis. From this point of view, it can be inferred that estrogen or progesterone is more influential to the gastric carcinogenesis than serum lipid per se.
The inverse relationship between cholesterol and gastric cancer has been suggested in many studied so far [3, 4, 8, 13, 14, 23, 24]. However, the reason for this relationship was not clearly evaluated. Traditionally, hypercholesterolemia, which is known to enhance oxidative stress and chronic inflammation [25,26,27], was expected to be carcinogenic. However, it was not true for many types of cancers. In the earlier studies, the strong relationships were more prominent during the first few years [8, 23, 24], thus reverse causation was suspected [28]. Nevertheless, more recent studies confirmed the true inverse relationship with sensitivity analysis by excluding those who developed gastric cancer in the first few years of the follow-up period [3, 4, 13]. However, such inverse association was not always true among women. Considering our results, the inconsistent association between cholesterol and gastric cancer among women in previous researches seems to be because of their different composition of pre- and postmenopausal women.
Female sex hormone is largely known to be protective against gastric carcinogenesis. Gastric cancer is male predominant worldwide and the male-to-female ratio is almost consistent globally regardless of populations with low and high gastric cancer incidence, and which was not changed for decades despite the global decrease of gastric cancer incidence [16]. The age-specific male-to-female ratio curve shows 10–15-year delay of the gastric cancer development, and which indicates the protective effect of female sex hormone. In menopausal women, the estrogen level is almost equal to that of men, and similarly their risks for gastric cancer are almost the same [29]. A previous study, which analyzed male prostate cancer patients who were treated with high-dose estrogen, demonstrated lower incidence of gastric cancer compared with general male population [17]. Many researches about menstrual and reproductive data showed that larger exposure to estrogen is related with lower risk of gastric cancer [30,31,32,33,34]. A research reported increased incidence of gastric cancer in tamoxifen, the anti-estrogen, users [35]. In animal studies, oophorectomy was related with increased risk of gastric cancer [18], and castration and administration of estrogen in male rats showed protective effect against gastric cancer [36, 37].
The exact mechanism how estrogen displays protective role against gastric cancer is unclear. However, estrogen is known to exert anti-inflammatory effect [19,20,21], antioxidant effect [22], and cell cycle regulation [38], which thereby is thought to display tumor suppressor function. Such regulatory function is displayed through estrogen receptors in various tissues in both men and women, and estrogen receptors are found in normal gastric mucosa as well as gastric cancer tissue [39,40,41,42]. Also it has been reported that estrogen increases apoptosis in gastric cancer cells [43].
In postmenopausal women, estrogen level is similar to that of men. In such relative estrogen depletion status, high cholesterol level was shown to be protective against gastric cancer. A possible explanation is that those with high cholesterol level may have relatively high estrogen level. In the depletion of gonadal estrogen secretion, estrogen is mainly produced by aromatase in adipose tissue, and cholesterol is the major precursor of the estrogen. According to a research, among postmenopausal women, obesity was related with relatively higher estrogen level [44]. This might be because of their abundant aromatase in the adipose tissue, and obesity is highly related with hypercholesterolemia as well. Also in an animal experiment, low cholesterol level induced by lipid-lowering agents decreased estrogen and progesterone level and induced reproductive dysfunction [45]. Thus, it can be inferred that hypercholesterolemia exerts protective effect against gastric cancer through higher level of estrogen which is synthesized mainly using the cholesterol. As can be the same situation in male. Another possibility is that cholesterol itself is directly related with low-risk of gastric cancer somehow, although not as potent as estrogen. A previous study has demonstrated that Helicobacter pylori metabolizes cholesterol to induce gastritis through C-type lectin receptors [46]. A recent study also reported that H. pylori depletes cholesterol from host cell membrane, which is necessary for T cell-mediated immune reaction, to evade full elimination by host immunity, resulting in chronic inflammatory condition [47]. Also, in another study, cholesterol-rich diet was shown to lead to a reduction of H. pylori load [48]. Therefore, cholesterol-rich condition might result in the reduction of gastric cancer risk by ameliorating H. pylori-induced chronic inflammation. However, this appears to play a minor role compared to estrogen.
In this study, statin medication was associated with lower gastric cancer risk among postmenopausal women, while not among premenopausal women. This was true under adjustment with cholesterol level. Traditionally, numerous evidences have supported statin’s risk-lowering effect against gastric cancer in preclinical and clinical studies [49,50,51,52,53,54]. However, the protective role of stain is thought to be unrelated with cholesterol lowering per se. It can be speculated that the protective effect of estrogen is much stronger than that of statins in premenopausal women with high blood estrogen levels.
However under age stratification, those under 55 years of age among postmenopausal women failed to show inverse relationship between lipid levels and gastric cancer risk. It can be inferred that this age group has yet to escape the protective effects of estrogen as the menopause period is relatively short.
In this study, TG showed inconsistent results regarding gastric cancer risk. This result was similar to previous researches [55, 56]. This might be because TG is not a direct precursor of sex hormone.
Current result showed that HDL-C has protective effect against gastric cancer even among those under HRT, while total cholesterol and LDL-C does not. Virtually, it is difficult to define the hormonal status of those under HRT. The artificial supplement can be insufficient compared with natural secretion. Also, their hormonal status can be even more complicated because of the various types of HRT, including estrogen only or a combination of estrogen and progesterone. Nonetheless, it can be translated at least that HDL-C somehow has significant effect even in relative female sex hormonal depletion state. Also, among those without HRT, HDL-C had slightly stronger risk-lowering effect and stronger relationship than LDL-C. Although little has been studied about the relationship between HDL-C and gastric cancer, a previous study reported lower HDL-C was associated with higher incidence of gastric dysplasia [57].
This study has several limitations. First, we could not analyze the direct relationship between estrogen and gastric cancer as the estrogen level was not achievable. We think that this should be investigated in future studies. Second, there might be someone in the premenopausal group who experienced menopause during the follow-up period. As we only defined the groups according to the menopausal status at the time of enrollment, we could not take such changes into account in the analysis. Third, serum lipids were only measured at baseline. Considering intra-individual variation, we might have misclassified some individuals in our population. However, we believe our result still has importance as it showed statistically significant relationship despite including such time-varying factors. Such misclassifications, if any, would have altered the trend towards null effect or attenuated the relationship. Thus the association observed in this study is likely to be reliable. Forth, we could not evaluate any differences in the different histological types of gastric cancer because the NHIC dataset lacks such information. Depending on gastric cancer histology, different results may be shown. Thus, further research is necessary to clarify this issue. Lastly, we could not adjust for H. pylori infection status. In previous researches, it has been reported that H. pylori infection is associated with lower HDL-C and higher total cholesterol, LDL-C, and TG [58,59,60,61,62]. However, the fact that total cholesterol and LDL-C which are positive correlators of H. pylori showed inverse association with gastric cancer development among postmenopausal women demonstrates their own gastric cancer-preventing effect. Regarding HDL-C, it has been shown in a previous large-scale cohort study that HDL-C is inversely related with gastric cancer risk even under adjustment with H. pylori infection status [62]. Furthermore, there is no reason for the association between H. pylori and lipid to be different in pre- and postmenopausal women.
In conclusion, our population-based cohort study demonstrated that cholesterol is inversely associated with the risk of gastric cancer among postmenopausal women, just as in men. Therefore, we suggest that it would be better not to control cholesterol too tightly after menopause in terms of gastric cancer prevention. There should be a more tailored cholesterol control guideline, especially suitable for postmenopausal women.
References
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–360.
Karr S. Epidemiology and management of hyperlipidemia. Am J Manag Care. 2017;23:S139–48.
Iso H, Ikeda A, Inoue M, Sato S, Tsugane S, JPHC Study Group. Serum cholesterol levels in relation to the incidence of cancer: The JPHC study cohorts. Int J Cancer. 2009;125:2679–86.
Kitahara CM, Berrington de González A, Freedman ND, Huxley R, Mok Y, Jee SH, et al. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol. 2011;29:1592–8.
Wulaningsih W, Garmo H, Holmberg L, Hammar N, Jungner I, Walldius G, et al. Serum lipids and the risk of gastrointestinal malignancies in the Swedish AMORIS study. J Cancer Epidemiol. 2012;2012:792034.
Knekt P, Reunanen A, Aromaa A, Heliövaara M, Hakulinen T, Hakama M. Serum cholesterol and risk of cancer in a cohort of 39,000 men and women. J Clin Epidemiol. 1988;41:519–30.
Schatzkin A, Hoover RN, Tayler PR, Ziegler RG, Carter CL, Albanes D, et al. Site-specific analysis of total serum cholesterol and incident cancer in the national health and nutrition examination survey i epidemiologic follow-up study. Cancer Res. 1988;48:452–8.
Törnberg SA, Holm LE, Carstensen JM, Eklund GA. Cancer incidence and cancer mortality in relation to serum cholesterol. J Natl Cancer Inst. 1989;81:1917–21.
Law MR, Thompson SG. Low serum cholesterol and the risk of cancer: an analysis of the published prospective studies. Cancer Causes Control. 1991;2:253–61.
Delahaye F, Bruckert E, Thomas D, Emmerich J, Richard JL. Serum cholesterol and cancer. Is there a causal relationship? Arch Mal Coeur Vaiss. 1992;85:37–45.
Strasak AM, Pfeiffer RM, Brant LJ, Rapp K, Hilbe W, Oberaigner W, et al. Time-dependent association of total serum cholesterol and cancer incidence in a cohort of 172,210 men and women: a prospective 19-year follow-up study. Ann Oncol. 2009;20:1113–20.
Ahn J, Lim U, Weinstein SJ, Schatzkin A, Hayes RB, Virtamo J, et al. Prediagnostic total and high-density lipoprotein cholesterol and risk of cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:2814–21.
Asano K, Kubo M, Yonemoto K, Doi Y, Ninomiya T, Tanizaki Y, et al. Impact of serum total cholesterol on the incidence of gastric cancer in a population-based prospective study: the Hisayama study. Int J Cancer. 2008;122:909–14.
Törnberg SA, Carstensen JM, Holm LE. Risk of stomach cancer in association with serum cholesterol and beta-lipoprotein. Acta Oncl. 1988;27:39–42.
Patel S, Homaei A, Raju AB, Meher BR. Estrogen: The necessary evil for human health, and ways to tame it. Biomed Pharmacother. 2018;102:403–11.
Sipponen P, Correa P. Delayed rise in incidence of gastric cancer in females results in unique sex ration (M/F) pattern: etiologic hypothesis. Gastric Cancer. 2002;5:213–9.
Lindblad M, Ye W, Rubio C, Lagergren J. Estrogen and risk of gastric cancer: a protective effect in a nationwide cohort study of patients with prostate cancer in Sweden. Cancer Epidemiol Biomarkers Prev. 2004;13:2203–7.
Freedman ND, Cho WH, Gao YT, Shu XO, Ji BT, Yang G, et al. Menstrual and reproductive factors and gastric cancer risk in a large prospective study of women. Gut. 2007;56:1671–7.
Pfeilschifter J, Koditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23:90–119.
Kalaitzidis D, Glimore TD. Transcription factor cross-talk: the estrogen receptor and NF-kappaB. Trends Endoscrinol Metab. 2005;16:46–52.
De Bosscher K, Vanden BW, Haegeman G. Cross-talk between nuclear receptors and nuclear factor kappaB. Oncogene. 2006;25:6868–86.
Speir E, Yu ZX, Takeda K, Ferrans VJ, Cannon RO. Antioxidant effect of estrogen on cytomegalovirus-induced gene expression in coronary artery smooth muscle cells. Circulation. 2000;102:2990–6.
Sherwin RW, Wentworth DN, Cutler JA, Hulley SB, Kuller LH, Stamler J. Serum cholesterol levels and cancer mortality in 361,662 men screened for the multiple risk factor intervention trial. JAMA. 1987;257:943–8.
Song YM, Sung J, Kim JS. Which cholesterol level is related to the lowest mortality in population with low mean cholesterol level: a 64-year follow-up study of 482,472 Korean men. Am J Epidemiol. 2000;151:739–47.
Erdelyi I, Levenkova N, Lin EY, Pinto JT, Lipkin M, Quimby FW, et al. Western-style diets induce oxidative stress and dysregulate immune responses in the colon in a mouse model of sporadic colon cancer. J Nutr. 2009;139:2072–8.
Ahotupa M, Suomela JP, Vuorimaa T, Vasankari T, et al. Lipoprotein-specific transport of circulating lipid peroxides. Ann Med. 2010;42:521–9.
Moustafa T, Fickert P, Magnes C, Guelly C, Thueringer A, Frank S, et al. Alterations in lipid metabolism mediate inflammation, fibrosis, and proliferation in a mouse model of chronic cholestatic liver injury. Gastroenterol. 2012;142:140–51.
Iribarren C, Reed DM, Chen R, Yano K, Dwyer JH. Low serum cholesterol and mortality: which is the cause and which is the effect? Circulation. 1995;92:2396–403.
Chen C, Gong X, Yang X, Shang X, Du Q, Liao Q, et al. The roles of estrogen and estrogen receptors in gastrointestinal disease (Review). Oncol Let. 2019;18:5673–80.
Palli D, Cipriani F, Decarli A, Galli M, Saieva C, Fraumeni JF Jr, et al. Reproductive history and gastric cancer among postmenopausal women. Int J Cancer. 1994;56:812–5.
Frise S, Kreiger N, Gallinger S, Tomlinson G, Cotterchio M. Menstrual and reproductive risk factors and risk for gastric adenocarcinoma in women: findings from the Canadian national enhanced cancer surveillance system. Ann Epidemiol. 2006;12:908–16.
La Vecchia C, D’Avanzo B, Franceschi S, Negri E, Parazzini F, Decarli A. Menstrual and reproductive factors and gastric-cancer risk in women. Int J Cancer. 1994;59:761–4.
Kaneko S, Tamakoshi A, Ohno Y, Mizoue T, Yoshimura T, JACC Study Group. Menstrual and reproductive factors and the mortality risk of gastric cancer in Japanese menopausal females. Cancer Causes Control. 2003;14:53–9.
Inoue M, Ito LS, Tajima K, Yamamura Y, Kodera Y, Takezaki T, et al. Height, weight, menstrual and reproductive factors and risk of gastric cancer among Japanese postmenopausal women: analysis by subsite and histologic subtype. Int J Cancer. 2002;97:833–8.
Rutqvist LE, Johansson H, Signomklao T, Johansson U, Fornander T, Wilking N. Adjuvant tamoxifen therapy for early stage breast cancer and second primary malignancies. Stockholm Breast Cancer Study Group. J Natl Cancer Inst. 1995;87:645–51.
Furukawa H, Iwanaga T, Koyana H, Taniguchi H. Effect of sex hormones on the experimental induction of cancer in rat stomach-a preliminary study. Digestion. 1982;23:151–5.
Campbell-Thomson M, Lauwers GY, Reyher KK, Cromwell J, Shiverick KT. 17Beta-Estradiol modulates gastroduodenal preneoplastic alterations in rats exposed to the carcinogen N-methyl-N’-nitro-nitrosoguanidine. Endocrinology. 1999;140:4886–94.
Katzenellenbogen BS. Estrogen receptors: bioactivities and interactions with cell signaling pathways. Bio Reprod. 1996;54:287–93.
Tokunaga A, Nishi K, Matsukura N, Tanaka N, Onda M, Shirota A, et al. Estrogen and progesterone receptors in gastric cancer. Cancer. 1986;57:1376–9.
Karat D, Brotherick I, Shenton BK, Scott D, Raimes SA, Griffin SM. Expression of oestrogen and progesteron receptors in gastric cancer: a flow cytometric study. Br J Cancer. 1999;80:1271–4.
Zhao XH, Gu SZ, Liu SX, Pan BR. Expression of estrogen receptor and estrogen receptor messenger RNA in gastric carcinoma tissues. World J Gastroenterol. 2003;9:665–9.
Takano N, Iizuka N, Hazama S, Yoshino S, Tangoku A, Oka M. Expression of estrogen receptor-alpha and -beta mRNAs in human gastric cancer. Cancer Lett. 2002;176:129–35.
Pricci M, Linsalata M, Russo F, Messa C, Amati L, Caradonna L, et al. Effects of 17β-estradiol administration on apoptosis and polyamine content in AGS cell line. Anticancer Res. 2001;21:3215–20.
Key TJ, Allen NE, Verkasalo PK, Banks E. Energy balance and cancer: the role of sex hormones. Proc Nutr Soc. 2001;60:81–9.
Shambharkar AV, Varshney VP, Agarwal N, Agarwal SK, Sanwal PC, Pande JK. Changes in serum triiodothyronine, thyroxine, estradiol-17 beta, progesterone levels and egg production in hypocholesterolemia induced Japanese quails Coturnix coturnix japanica. Indian J Exp Biol. 1996;34:987–90.
Nagata M, Toyonaga K, Ishikawa E, Haji S, Okahashi N, Takahashi M, et al. Helicobacter pylori metabolites exacerbate gastritis through C-type lectin receptors. J Exp Med. 2020;218:e20200815.
Morey P, Pfannkuch L, Pang E, Boccellato F, Sigal M, Imai-Matsushima A, et al. Helicobacter pylori deplets cholesterol in gastric glands to prevent interferon gamma signaling and escape the inflammatory response. Gastroenterol. 2018;154:1391–404.
Wunder C, Churin Y, Winau F, Warnecke D, Vieth M, Lindner B, et al. Cholesterol glucosylation promotes immune evasion by Helicobacter pylori. Nat Med. 2006;12:1030–8.
Follet J, Corcos L, Baffet G, Ezan F, Morel F, Simon B, et al. The association of statins and taxanes: an efficient combination trigger of cancer cell apoptosis. Br J Cancer. 2012;106:685–92.
Chushi L, Wei W, Kangkang X, Yongzeng F, Ning X, Xiaolei C. HMGCR is up-regulated in gastric cancer and promotes the growth and migration of the cancer cells. Gene. 2016;587:42–7.
Chiu HF, Ho SC, Chang CC, Wu TN, Yang CY. Statins are associated with a reduced risk of gastric cancer: a population-based case-control study. Am J Gastroenterol. 2011;106:2098–103.
Singh PP, Singh S. Statins are associated with reduced risk of gastric cancer: a systematic review and meta-analysis. Ann Oncol. 2013;24:1721–30.
Lin CJ, Liao WC, Lin HJ, Hsu YM, Lin CL, Chen YA, et al. Statins attenuate helicobacter pylori CagA translocation and reduce incidence of gastric cancer: in vitro and population-based case-control studies. PLoS ONE. 2016;11:e0146432.
Joo MK, Park JJ, Chun HJ. Additional benefits of routine drugs on gastrointestinal cancer: statins, metformin, and Proton Pump Inhibitors. Dig Dis. 2018;36:1–14.
Ulmer H, Borena W, Rapp K, Klenk J, Strasak A, Diem G, et al. Serum triglyceride concentrations and cancer risk in a large cohort study in Austria. Br J Cancer. 2009;101:1202–6.
Borena W, Stocks T, Jonsson H, Strohmaier S, Nagel G, Bjørge T, et al. Serum triglycerides and cancer risk in the metabolic syndrome and cancer (Me-Can) collaborative study. Cancer Cause Control. 2011;22:291–9.
Jung MK, Jeon SW, Cho CM, Tak WY, Kweon YO, Kim SK, et al. Hyperglycemia, hypercholesterolemia and the risk for developing gastric dysplasia. Dig Liver Dis. 2008;40:361–5.
Niemelä S, Karttunen T, Korhonen T, Läärä E, Karttunen R, Ikäheimo M, et al. Could Helicobacter pylori infection increase the risk of coronary heart disease by modifying serum lipid concentrations? Heart. 1996;75:573–5.
Laurila A, Bloigu A, Näyhä S, Hassi J, Leinonen M, Saikku P. Association of Helicobacter pylori infection with elevated serum lipids. Atherosclerosis. 1999;142:207–10.
Satoh H, Saijo Y, Yoshioka E, Tsutsui H. Helicobacter pylori infection is a significant risk for modified lipid profile in Japanese male subjects. J Atheroscler Thromb. 2010;17:1041–8.
Kim HL, Jeon HH, Park IY, Choi JM, Kang JS, Min KW. Helicobacter pylori infection is associated with elevated low density lipoprotein cholesterol levels in elderly Koreans. J Korean Med Sci. 2011;26:654–8.
Nam SY, Ryu KH, Park BJ, Park S. Effects of Helicobacter pylori infection and its eradication on lipid profiles and cardiovascular diseases. Helicobacter. 2015;20:125–32.
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number : HI18C1140).
Author information
Authors and Affiliations
Contributions
JHL, CMS, KDH, JY: study conception and design, data analysis/interpretation; JHL: manuscript drafting; EHJ, YJC, DHL: critical revision of manuscript; All authors read and approved the final manuscript and the authorship list. All authors fulfill the ICMJE criteria for authorship.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1996 and later versions.
Informed consent
Formal consent was not obtained because of the retrospective nature or this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lim, J.H., Shin, C.M., Han, K. et al. Nationwide cohort study: cholesterol level is inversely related with the risk of gastric cancer among postmenopausal women. Gastric Cancer 25, 11–21 (2022). https://doi.org/10.1007/s10120-021-01241-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-021-01241-1