Abstract
Background
Despite recent progress in systemic chemotherapy, the prognosis of gastric cancer patients with peritoneal metastasis (P1) or positive peritoneal cytology findings (CY1) is still poor. We developed a regimen combining intraperitoneal (IP) paclitaxel (PTX) with S-1 and PTX, which can produce notable efficacy with regard to peritoneal lesions. Surgery after response to combination chemotherapy is a promising option for P1 or CY1 gastric cancer. A retrospective study was performed to evaluate the safety and efficacy.
Methods
This study enrolled 100 primary P1 or CY1 gastric cancer patients treated with IP PTX plus S-1 and PTX at the University of Tokyo Hospital between 2005 and 2011. Radical gastrectomy was performed when peritoneal cytology findings became negative, and the disappearance or obvious shrinkage of peritoneal metastasis was confirmed by laparoscopy. The same chemotherapy regimen was restarted after surgery and repeated with appropriate dose reduction.
Results
Gastrectomy was performed in 64 (P1 56, P0CY1 8) of 100 (P1 90, P0CY1 10) patients. R0 resection was achieved in 44 patients (69%). The median survival time was 30.5 months [95% confidence interval (CI) 23.6–37.7 months] from the initiation of intraperitoneal chemotherapy and 34.6 months (95% CI 26.8–39.4 months) from the diagnosis of gastric cancer. Postoperative complications included anastomotic leakage and pancreatic fistula, each in two patients, which were cured conservatively. There were no treatment-related deaths. The median survival time of the 36 patients who did not undergo surgery was 14.3 months (95% CI 10.0–17.8 months).
Conclusions
Surgery after response to intraperitoneal and systemic chemotherapy is safe and may prolong the survival of P1 and CY1 gastric cancer patients.
Similar content being viewed by others
Introduction
The standard of care for gastric cancer patients with distant metastasis is systemic chemotherapy [1, 2]. According to the results of pivotal clinical trials [2–5], the combination of S-1 (tegafur, gimeracil, oteracil) or capecitabine with cisplatin or oxaliplatin is recommended for first-line chemotherapy, and paclitaxel (PTX) plus ramucirumab is recommended for second-line chemotherapy by the Japanese gastric cancer treatment guidelines [1]. Recent progress in systemic chemotherapy has improved the prognosis of patients, but the median survival time (MST) has been prolonged to only approximately 1 year [2–6]. Multidisciplinary treatment combining chemotherapy and surgery is now regarded as a promising option because metastatic lesions apparently disappear or shrink considerably after chemotherapy in some patients. However, the treatment strategy of gastrectomy followed by chemotherapy failed to provide a survival advantage compared with chemotherapy alone, probably because of impaired adherence to chemotherapy after gastrectomy [7]. Another multidisciplinary strategy is conversion therapy, which is defined as a surgical treatment aiming at an R0 resection after chemotherapy for tumors that were originally unresectable or marginally resectable for technical and/or oncological reasons [8]. Conversion therapy has advantages in that chemotherapy is administered to patients with better general conditions and surgery is performed only on responders to chemotherapy.
Gastric cancer patients with peritoneal metastasis (P1) or positive peritoneal cytology findings (CY1) are generally treated with systemic chemotherapy as patients with other distant metastasis are. However, considering the unique mode of tumor spreading in peritoneal metastasis, it may be reasonable to approach the metastatic lesions and free cancer cells in the peritoneal cavity directly with intraperitoneal chemotherapy. IP PTX provides a high local concentration over a long time because of its pharmacokinetic properties [9], and the effects on peritoneal metastasis have been verified by clinical trials in ovarian cancer [10] and preliminarily reported in gastric cancer [11–13]. We designed a regimen combining weekly IP PTX with S-1 and PTX for gastric cancer, and we determined the recommended dose of IP PTX to be 20 mg/m2 in a phase I trial [14]. In our phase II trials, the 1-year overall survival rates were 78% in P1 or CY1 patients [15] and 77% in P1 patients [16]. In our phase III trial, the MST was 17.7 months [95% confidence interval (CI) 14.7–21.5 months] [17]. In these trials, the overall response rate was 53–71% and the amount of malignant ascites decreased in 62–86% of patients, whereas grade 3/4 neutropenia was observed in 34–50% of patients and other adverse events were relatively mild [15–17]. Some of the patients treated with IP PTX showed disappearance or obvious shrinkage of peritoneal metastasis after chemotherapy, whereas the primary tumor was difficult to control for months, which encouraged us to perform surgery after response to chemotherapy [18]. A retrospective study was performed in patients including those enrolled in the phase I and phase II trials [14–16] to evaluate the safety and efficacy of surgery after response to systemic and intraperitoneal chemotherapy.
Patients and methods
Patients
This retrospective study enrolled 100 primary gastric cancer patients with peritoneal metastasis and/or positive peritoneal cytology findings treated with IP PTX plus S-1 and PTX at the University of Tokyo Hospital between 2005 and 2011. Patients who underwent noncurative gastrectomy for palliation before initiation of intraperitoneal chemotherapy were not enrolled in this study.
Treatment
Patients underwent diagnostic laparoscopy with peritoneal cytology and an intraperitoneal port was implanted in the subcutaneous space of the lower abdomen, with a catheter placed in the pelvic cavity. Combination chemotherapy of IP PTX plus S-1 and PTX was initiated approximately 7 days after laparoscopy. S-1 was administered orally twice daily at 80 mg/m2/day for 14 consecutive days, followed by 7 days of rest. PTX was administered intravenously at 50 mg/m2 and intraperitoneally at 20 mg/m2 on days 1 and 8. PTX was diluted in 1 L of normal saline and administered through an intraperitoneal port in 1 h concurrently with intravenous infusion after standard premedication for PTX. The treatment course was repeated every 3 weeks mainly in the outpatient department until disease progression or unacceptable toxicity. Tumor responses were evaluated every three courses by computed tomography and upper gastrointestinal tract endoscopy. Cytology of ascites or peritoneal lavage fluid collected through an intraperitoneal port was performed by Papanicolaou staining at the end of each course.
Gastrectomy was considered when a remarkable response to combination chemotherapy was observed in patients who would tolerate surgery. The indication criteria for surgery were negative peritoneal cytology findings, the disappearance or obvious shrinkage of peritoneal metastasis, and no unresectable metastasis identified by diagnostic imaging. The response of the peritoneal metastasis was evaluated by second-look laparoscopy, the timing of which was determined by consideration of the extent of peritoneal metastasis before chemotherapy and the response to chemotherapy. Gastrectomy with lymph node dissection was performed when the tumor was resectable by standard or extended gastrectomy excluding pancreaticoduodenectomy or thoracotomy. Splenectomy was performed in patients with suspected splenic hilar lymph node metastasis and/or peritoneal metastasis on the gastrosplenic ligament. The distal part of the pancreas, colon, small intestine, or adnexa was resected when direct invasion of the primary tumor or metastasis was observed. When white nodules or scar-like lesions remained on the parietal peritoneum or mesentery, they were resected or ablated as much as possible with an electric scalpel. Neither extended peritonectomy nor intraoperative intraperitoneal chemotherapy with or without hyperthermia was performed. The same combination chemotherapy regimen was restarted after surgery as soon as possible with appropriate dose reduction. The intensity and duration of chemotherapy were determined by attending physicians in each case considering the curability of surgery and postoperative decreases in chemotherapy tolerance.
Assessment and statistical methods
The baseline characteristics of patients who underwent surgery and those who did not undergo surgery were compared by Pearsonʼs chi square test or Fisherʼs exact test when appropriate. The extent of peritoneal metastasis was classified according to the Japanese classification of gastric carcinoma 12th edition and 1st English edition [19]: P1, metastases immediately adjacent to the stomach; P2, several scattered metastases within the peritoneal cavity; and P3, numerous metastases throughout the peritoneal cavity. The operating procedure, postoperative complications, residual tumor status, and histological response were evaluated. The histological response of the primary tumor was classified according to the Japanese classification of gastric carcinoma 14th edition and 3rd English edition [20]: grade 0, no evidence of effect; grade 1a, viable tumor cells occupy two thirds or more of the tumorous area; grade 1b viable tumor cells occupy from one third to less than two thirds of the tumorous area; grade 2, viable tumor cells occupy less than one third of the tumorous area; and grade 3, no viable tumor cells.
The overall survival rates were estimated by the Kaplan–Meier method. The overall survival rates of groups divided according to the extent of peritoneal metastasis, residual tumor status, or histological response were compared with the log-rank test. P < 0.05 was considered statistically significant, with Bonferroni correction for multiple comparisons between the three groups. All statistical analyses were performed with JMP Pro version 11.0.0 (SAS Institute, Cary, NC, USA).
Results
Intraperitoneal and systemic chemotherapy
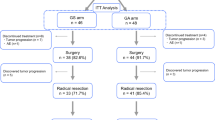
Among 100 P1 and/or CY1 gastric cancer patients, 68 showed remarkable clinical response and were considered for surgery. Excluding one 86-year-old patient who declined surgery, second-look laparoscopy was performed in 67 patients, and the disappearance or obvious shrinkage of peritoneal metastasis was observed in 65 patients. Laparotomy was performed in these 65 patients, and gastrectomy was performed in 64 patients, excluding one patient with a primary tumor invading the pancreas and duodenum.
Patient characteristics
The clinical characteristics of the patients at the time of before intraperitoneal and systemic chemotherapy are shown in Table 1. Most of the patients had an Eastern Cooperative Oncology Group performance status of 0 or 1. Systemic chemotherapy had previously been administered in 43 patients. Among them, 39 patients had received S-1-based chemotherapy: S-1 and cisplatin in 25 patients, S-1 and taxane in 8 patients, and S-1 monotherapy in 6 patients. Many of the patients had type 4 tumors with undifferentiated histological type. The characteristics were similar among groups in terms of age, sex, receipt of previous chemotherapy, macroscopic type and histological type. However, patients who underwent surgery had significantly better Eastern Cooperative Oncology Group performance status and fewer peritoneal metastases compared with patients who did not undergo surgery. Of the 64 patients who underwent surgery, 53 patients (83%) had metastasis to the distant peritoneum (P2 or P3), and many had complications related to peritoneal metastasis: ascites in 36 patients, intestinal obstruction in 7 patients, and hydronephrosis in 6 patients. Eighty-one patients had no other distant metastasis, whereas 15 had metastasis to the ovary and 4 patients had metastasis to the para-aortic lymph nodes.
Surgical outcomes
Patients were given combination chemotherapy for a median of four courses (range 2–18 courses) before surgery. A summary of the types of surgery and the outcomes is given in Table 2. Total gastrectomy was performed in 58 patients (91%). Splenectomy was performed in 19 patients, and distal pancreatectomy was performed in 3 patients with tumors invading the pancreas. Partial or extended colectomy was performed in 13 patients with peritoneal metastasis on the serosa or in the wall of the colon. Adnexectomy was performed in eight patients with ovarian metastasis. The extent of lymph node dissection was D1 in 37 patients, for whom the prophylactic dissection of splenic hilar lymph nodes was omitted, D2 in 26 patients, and D3 in 1 patient with para-aortic lymph node metastasis. The median number of lymph nodes dissected was 35 (range 8–101). The median operation time was 296 min (range 165–550 min). The median blood loss was 685 ml (range 130–2340 ml), and a blood transfusion was performed in 11 patients. Postoperative complications severer than Clavien–Dindo grade I included anastomotic leakage and pancreatic fistula, each in two patients, all of which were grade II and cured conservatively. There were no treatment-related deaths.
After surgery, 44 patients (69%) had no residual tumor either macroscopically or microscopically (R0). The pathological examination revealed cancer cells in the resection stump in six patients and biopsied scar-like lesions on the peritoneum in four patients (R1). In ten patients, numerous metastatic nodules had shrunk remarkably after chemotherapy, but it was difficult to remove all the visible nodules during surgery (R2).
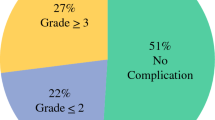
Histological examination of the resected primary tumor showed necrosis or disappearance of two thirds or more of the tumor in 16 patients (25%). The median number of metastatic lymph nodes was 4 (range 0–67). The numbers of patients with N0, N1, N2, N3a, and N3b metastasis were 12, 14, 14, 9, and 15 respectively. Splenic hilar lymph node metastasis was found in 5 of 19 patients who underwent splenectomy.
Survival
The MST of all 100 patients was 20.6 months (95% CI 17.3–27.7 months). The MST of the 64 patients who underwent surgery was 30.5 months (95% CI 23.6–37.7 months) from the initiation of intraperitoneal chemotherapy (Fig. 1) and 34.6 months (95% CI 26.8–39.4 months) when it was calculated from the diagnosis of gastric cancer. From the date of surgery, the MST was 25.6 months (95% CI 17.2–33.2 months) and the 1-year overall survival rate was 73.3% (95% CI 61.2–82.7%). The MSTs of patients with P0CY1 or P1, P2, and P3 metastasis were not reached (95% CI 22.1 months to not reached), 36.9 months (95% CI 23.6–39.9 months), and 23.8 months (95% CI 15.5–30.4 months) respectively (Fig. 2). Among these three groups, the difference was significant between P0CY1 or P1 and P3 patients (log-rank test, P = 0.0037). According to the residual tumor status, there was no significant difference in overall survival, although all the 5-year survivors were R0 patients (Fig. 3). No difference was observed according to the extent of lymph node dissection. According to the histological response, the overall survival was significantly longer in patients with a grade 2 or grade 3 response (viable tumor cells occupy less than one third of the tumorous area) than in patients with a grade 1a or grade 1b response (viable tumor cells occupy one third or more of the tumorous area) [MST 39.9 months (95% CI 34.5–79.7 months) vs 26.1 months (95% CI 20.0–30.8 months); log-rank test, P = 0.019] (Fig. 4). The MST of the 36 patients who did not undergo surgery was 14.3 months (95% CI 10.0–17.8 months) (Fig. 1).
Relapse or progression was observed in 58 of the 64 patients who underwent surgery, with a median time of 17.0 months (95% CI 13.8–24.6 months). The first site of recurrence or progression was the peritoneum in 38 patients and was another site in 26 patients (both sites in 6 patients). The site of metastasis other than the peritoneum was the lymph nodes in 13 patients, liver in 5 patients, bone in 4 patients, ovary in 3 patients, and pleura, adrenal gland, and meninges in 2 patients (more than one site in 4 patients). The site of recurrence or progression was not associated with the histological response in the primary tumor.
Discussion
We performed surgery on 64 of 100 P1 or CY1 gastric cancer patients treated with IP PTX plus S-1 and PTX, and we obtained promising results in terms of safety and efficacy. The results suggest that surgery after response to chemotherapy has clinical efficacy, although the MSTs of patients who underwent surgery and patients who did not undergo surgery are not comparable because of differences in baseline performance status, the extent of peritoneal metastasis, and response to chemotherapy.
This combination chemotherapy regimen would be much more effective for peritoneal metastasis than for a primary tumor or other metastasis because of a higher concentration in the peritoneal cavity [19]. We have experienced many patients in whom peritoneal metastasis had been controlled for years and the primary tumor progressed within months. Therefore, our rationale for surgery is to resect the primary tumor when peritoneal metastasis is well controlled and thereby prevent new metastasis, bleeding, and stenosis that may be caused by the primary tumor, which may lead to the prolongation of survival. In that sense our surgery, especially in P2 and P3 patients, might not be the definitive “conversion surgery” to intend curability, such as gastrectomy after S-1 and cisplatin therapy or docetaxel, cisplatin, and S-1 therapy in previous reports [21, 22]. Meanwhile, surgery might cause the progression of the residual disease, apparent or occult, because of the perioperative pausing of chemotherapy, a postoperative decrease in the tolerability of chemotherapy, and a postoperative reduction in antitumor immunity. Thus, surgery should be performed after peritoneal metastasis is sufficiently controlled, or eliminated if possible, and excessive surgical stress and postoperative complications should be avoided.
The indication criteria for surgery constitute the most crucial issue in this conversion therapy. In the early years, we used to confirm the disappearance of peritoneal metastasis not only macroscopically but also microscopically by multiple biopsies of the peritoneum before gastrectomy. After having experienced some patients with a favorable clinical course, we extended the criterion first to the macroscopic disappearance and then to the obvious shrinkage of peritoneal metastasis. Along with these modifications, ten patients had microscopic residual tumor (R1) and ten patients had macroscopic residual tumor (R2) after surgery, but there was no significant difference in overall survival compared with the patients with no residual tumor (R0) (Fig. 3). This result indicates that residual peritoneal tumors could be controlled by postoperative continuation of intraperitoneal and systemic chemotherapy in patients with a remarkable shrinkage of peritoneal lesions, suggesting the possible benefit of gastrectomy with our extended criterion. Regarding the histological response of the primary tumor, patients with a response of grade 2 or grade 3 had a significantly better prognosis than patients with a response of grade 1a or grade 1b (Fig. 4). Since peritoneal lesions were similarly controlled in those patients with a poor histological response, this suggests that the response in the primary site might be dependent mainly on the sensitivity to systemic chemotherapy. Therefore, it may be reasonable to change the postoperative regimen and use different systemic drugs with the same intraperitoneal chemotherapy regimen.
The timing of surgery is another important issue. The best timing would be when the tumor had shrunk the most by chemotherapy, which is impossible to know beforehand. On an empirical basis, we perform surgery on P0CY1 or P1, P2, and P3 patients after 3, 6, and 9–18 courses respectively, with modification considering the timing of negative conversion on peritoneal cytology, computed tomography findings, and changes in the levels of tumor markers, especially cancer antigen 125.
Judging from the results of this study, our overall strategy and treatment can be regarded as appropriate. However, some patients developed progression soon after surgery, and it is important to know for which population surgery was effective. It is clinically suggested that the extent of peritoneal metastasis and response to chemotherapy are associated with the prognosis of patients, but the analysis of prognostic factors is difficult at present because we have no accurate and precise method to classify or quantify these two factors.
In conclusion, surgery after response to intraperitoneal and systemic chemotherapy is safe and may prolong the survival of gastric cancer patients with peritoneal metastasis or positive peritoneal cytology findings. Since this is the retrospective study in a single institute, it will be necessary to perform a prospective randomized controlled trial or a large cohort study to verify the efficacy of surgery after response to chemotherapy. However, the randomization to surgery or the continuation of chemotherapy might be difficult for patients and physicians to accept. We would like to spread intraperitoneal chemotherapy and surgery after response to chemotherapy nationwide soon and clarify the role of surgery by analyzing the accumulated data in detail.
References
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2016. doi:10.1007/s10120-016-0622-4
PDQ Adult Treatment Editorial Board. Gastric cancer treatment (PDQ®)—health professional version. Bethesda: National Cancer Institute. 2016. http://www.cancer.gov/types/stomach/hp/stomach-treatment-pdq.
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–21.
Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666–73.
Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol. 2015;26:141–8.
Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–35.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97.
Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016;17:309–18.
Yoshida K, Yamaguchi K, Okumura N, Tanahashi T, Kodera Y. Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification. Gastric Cancer. 2016;19:329–38.
Markman M, Rowinsky E, Hakes T, Reichman B, Jones W, Lewis JL Jr, et al. Phase I trial of intraperitoneal taxol: a Gynecoloic Oncology Group study. J Clin Oncol. 1992;10:1485–91.
Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43.
Fushida S, Furui N, Kinami S, Ninomiya I, Fujimura T, Nishimura G, et al. Pharmacologic study of intraperitoneal paclitaxel in gastric cancer patients with peritoneal dissemination. Gan To Kagaku Ryoho. 2002;29:2164–7.
Kodera Y, Ito Y, Ito S, Ohashi N, Mochizuki Y, Yamamura Y, et al. Intraperitoneal paclitaxel: a possible impact of regional delivery for prevention of peritoneal carcinomatosis in patients with gastric carcinoma. Hepatogastroenterology. 2007;54:960–3.
Imano M, Peng YF, Itoh T, Nishikawa M, Satou T, Yasuda A, et al. A preliminary study of single intraperitoneal administration of paclitaxel followed by sequential systemic chemotherapy with S-1 plus paclitaxel for advanced gastric cancer with peritoneal metastasis. Anticancer Res. 2012;32:4071–5.
Ishigami H, Kitayama J, Otani K, Kamei T, Soma D, Miyato H, et al. Phase I pharmacokinetic study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer. Oncology. 2009;76:311–4.
Ishigami H, Kitayama J, Kaisaki S, Hidemura A, Kato M, Otani K, et al. Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol. 2010;21:67–70.
Yamaguchi H, Kitayama J, Ishigami H, Emoto S, Yamashita H, Watanabe T, et al. A phase 2 trial of intravenous and intraperitoneal paclitaxel combined with S-1 for treatment of gastric cancer with macroscopic peritoneal metastasis. Cancer. 2013;119:3354–8.
Ishigami H, Fujiwara Y, Fukushima R, Nashimoto A, Yabusaki H, Imamoto H, et al. Phase III study of intraperitoneal paclitaxel plus S-1/paclitaxel compared with S-1/cisplatin in gastric cancer patients with peritoneal metastasis: PHOENIX-GC trial. J Clin Oncol. 2016;34:4014.
Kitayama J, Ishigami H, Yamaguchi H, Yamashita H, Emoto S, Kaisaki S, et al. Salvage gastrectomy after intravenous and intraperitoneal paclitaxel (PTX) administration with oral S-1 for peritoneal dissemination of advanced gastric cancer with malignant ascites. Ann Surg Oncol. 2014;21:539–46.
Japanese Research Society for Gastric Cancer. Japanese classification of gastric carcinoma. 1st English edition. Tokyo: Kanehara; 1995.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–12.
Tsuburaya A, Mizusawa J, Tanaka Y, Fukushima N, Nashimoto A, Sasako M. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg. 2014;101:653–60.
Kinoshita J, Fushida S, Tsukada T, Oyama K, Okamoto K, Makino I, et al. Efficacy of conversion gastrectomy following docetaxel, cisplatin, and S-1 therapy in potentially resectable stage IV gastric cancer. Eur J Surg Oncol. 2015;41:1354–60.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Japan Agency for Medical Research and Development.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent was obtained from all patients for their being included in the study.
Rights and permissions
About this article
Cite this article
Ishigami, H., Yamaguchi, H., Yamashita, H. et al. Surgery after intraperitoneal and systemic chemotherapy for gastric cancer with peritoneal metastasis or positive peritoneal cytology findings. Gastric Cancer 20 (Suppl 1), 128–134 (2017). https://doi.org/10.1007/s10120-016-0684-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-016-0684-3