Abstract
Background
Despite recent clinical trials, the sensitivity and resistance of metastatic gastric cancer to anti-HER2 and anti-EGFR therapy are still unclear.
Materials and methods
To clarify the HER2 and EGFR expression status in the metastatic sites, we immunohistochemically compared HER2 and EGFR expression between primary and metastatic tumors from 52 gastric cancer patients with liver metastases and 85 patients with peritoneal metastases.
Results
The HER2 positivity rate of primary and metastatic tumors in patients with liver metastases, especially with intestinal-type histology (70.6 and 80.0 %, respectively), was significantly higher than in primary and metastatic tumors (22.4 and 16.4 %, respectively) in patients with peritoneal metastases. HER2 positivity of the primary tumor and liver metastases showed good concordance (87.5 %) in patients with liver metastases. In contrast, the EGFR positivity rate of metastatic tumors (70.1 %) in patients with peritoneal metastases was significantly higher than that of metastatic tumors (37.5 %) in patients with liver metastases. HER2 and EGFR expression tended to be mutually exclusive, and HER2/EGFR double-positive cases were rare in patients with liver or peritoneal metastases. In four such patients with HER2/EGFR double-positive primary tumors, the HER2- and EGFR-positive areas were separate, and corresponding liver metastasis was only positive for HER2 and peritoneal metastasis only positive for EGFR.
Conclusion
These results indicate that HER2 and EGFR are preferentially expressed in the liver and peritoneal metastases, respectively, which would be potential targets for anti-HER2 and anti-EGFR molecular therapy in metastatic gastric cancer patients.
Similar content being viewed by others
Introduction
Globally, gastric cancer is the second highest cause of cancer-related deaths. The annual number of patients newly diagnosed with gastric cancer is more than 900,000 worldwide [1]. Despite the good prognosis of early gastric cancer, the prognosis of advanced gastric cancer is still poor, and the mean survival period after diagnosis is only about 10 months using current treatment modalities. Thus, molecular-targeting therapy is expected to be a potential alternative to conventional chemotherapy for gastric cancers [2].
Among various targeting molecules, EGFR and HER2 are well recognized as the most promising molecular targets for gastrointestinal, breast and lung cancers. In breast cancers, trastuzumab, a humanized monoclonal antibody targeted at the extracellular domain (IV) of HER2 [3], is now used worldwide as a standard molecular targeting therapy against HER2-positive metastatic breast cancer patients and as an adjuvant therapy for postoperative breast cancer patients [4]. Another anti-HER2 drug, lapatinib, which is a dual tyrosine kinase inhibitor that inhibits phosphorylation of both EGFR and HER2, has also been found to be clinically effective in patients with HER2-positive metastatic breast cancer [5]. Furthermore, the anti-EGFR antibody, cetuximab, has been proved to have clinically significant anti-tumor activity against advanced colorectal cancers and head and neck cancers [6].
In the gastric cancer field, a recent international prospective randomized phase III trial (TOGA study) demonstrated significantly prolonged overall survival with chemotherapy plus trastuzumab treatment compared with chemotherapy alone in HER2-positive metastatic gastric cancer patients [7, 8]. In contrast, as for EGFR-targeting drugs, a phase III clinical study of cetuximab in combination with cisplatin and capecitabine as a first-line treatment for patients with advanced gastric adenocarcinoma showed that cetuximab could not significantly increase progression-free survival in advanced gastric cancer patients [9]. Furthermore, the international phase II randomized clinical trial of gefitinib, an EGFR tyrosine kinase inhibitor, against stage IV gastric cancer in 75 patients showed that the overall therapeutic efficacy of gefitinib was quite low [10]. In these clinical trials of anti-EGFR agents with negative results, however, EGFR expression was not estimated for the decision to use anti-EGFR therapy. Therefore, the therapeutic activity of anti-EGFR agents against EGFR-overexpressing gastric cancers remains to be determined. Several investigators recently reported that EGFR positivity, but not HER2, was associated with poor patient outcomes after curative surgery in gastric cancers [11, 12]. Therefore, further clinical study for anti-EGFR agents with patient selection based on high EGFR expression is warranted.
Since the main targets of anti-HER2 and anti-EGFR therapy are metastatic tumors in stage IV gastric cancer patients, the expression status of EGFR and HER2 in the metastatic site is important for making the decision about the therapeutic strategy in metastatic gastric cancer patients. Detailed HER2 expression in the primary tumor with various stages and the concordance of HER2 expression between the primary tumor and general metastases have been reported in breast and gastric cancers [13, 14], but no detailed EGFR and HER2 expression focused on liver and peritoneal metastases has been reported in gastric cancers [15, 16]. In the present study, we investigated the EGFR and HER2 expression in the primary tumor and corresponding liver and peritoneal metastases, and in partly lymph node metastases, in patients with advanced gastric cancer and demonstrated their preferential HER2 and EGFR expression in the liver metastases and peritoneal metastases, respectively. Potential HER2 and EGFR targeting therapy in advanced gastric cancer will be discussed.
Materials and methods
Patients
A total of 137 gastric cancer patients were enrolled in this study. Patients underwent a biopsy, laparotomy or operation at the Department of Gastroenterological Surgery of Aichi Cancer Center Central Hospital between 1995 and 2000 and autopsy at the Division of Oncological Pathology of Aichi Cancer Center Research Institute between 2005 and 2011. The population included 85 patients with synchronous peritoneal metastases and 52 patients with liver metastases consisting of 20 patients with synchronous metastases and 32 patients with metachronous metastases. Seven patients with peritoneal metastasis showed synchronous liver metastasis, whereas patients with liver metastasis showed no simultaneous peritoneal metastasis. Specimens from gastric cancer patients with peritoneal metastases consisted of primary gastric cancer tissues (n = 85) and corresponding peritoneal metastatic tissues (n = 67). In 18 of the 85 patients with clinical peritoneal metastases, pathological specimens, but not cytological specimens from peritoneal metastases, could not be obtained because of no biopsy of metastatic sites. Similarly, in seven patients with dual peritoneal and liver metastases, liver metastatic tissues could not be obtained. On the other hand, specimens from gastric cancer patients with liver metastases consisted of primary gastric cancer tissues (n = 52) and corresponding liver metastatic tissues (n = 28). In 24 out of the 52 patients with clinical liver metastases, pathological specimens from liver metastases could not be obtained because of cases that were inoperable because of multiple liver metastases. Furthermore, 19 lymph node metastasis specimens were obtained from 24 patients with liver metastases. In 5 out of 24 patients, lymph node specimens could not be obtained because of the absence of lymph node metastasis.
The general patient characteristics are summarized in Table 1. Histology was simply subdivided into the intestinal type and diffuse type according to the Lauren classification. This study was approved by the institutional review board of Aichi Cancer Center.
Immunohistochemical staining for EGFR and HER2
Tissues were fixed in 10 % formalin for several days up to 3–4 days, embedded in paraffin and sectioned at 4 µm thickness for immunohistochemistry. Immunohistochemical staining of EGFR was carried out using the EGFR PharmDX staining kit (DakoCytomation, Carpinteria, CA) according to the manufacturer’s protocol. Briefly, after deparaffinization of the sections, enzymatic antigen retrieval was done in protease K solution for 5 min at room temperature, and then slides were covered for 5 min with hydrogen peroxidase to block endogenous peroxidase. Slides were incubated overnight at 4 °C with the primary mouse anti-EGFR MAb. The slides were then incubated with the detection system for 30 min. Staining was visualized with 0.01 % diaminobenzidine (DAB) for 5 min and counterstained in Meyer’s hematoxylin. Negative controls were performed using the mouse IgG1 MAb supplied with the kit. Positive (HT-29) and negative (CAMA-1) control cell lines were studied to validate each staining.
Immunohistochemical staining of HER2 was described previously [17]. Briefly, the sections were treated in a microwave at 98 °C for 10 min for antigen retrieval. After blocking nonspecific reactions, the sections were incubated at 4 °C overnight with rabbit polyclonal antibody to HER2 (DAKO Cytomation) diluted 1:300. After washing with PBS, the sections were incubated with biotinylated second antibodies for 30 min. The sections were washed again with PBS, then incubated with streptavidin-peroxidase complex (Vectastain ABC kit, Vector Laboratories, Burlingame, CA) for 60 min. The chromogen was developed with 0.01 % DAB, and the sections were counterstained with Meyer’s hematoxylin. The positive control cell line (GLM-1) was stained to validate staining.
DISH analysis
Dual-color in situ hybridization (DISH) was only conducted on several autopsy specimens fixed for 2 days. For DISH, hybridization was performed using a Ventana INFORM HER2 Dual ISH DNA Probe Cocktail (Roche Diagnostics K.K., Tokyo, Japan) as described previously [18]. HER2 is visualized as black signals by a dinitrophenyl (DNP)-labeled probe and ultraView SISH DNP detection kit. The Chr17 centromere is targeted with a digoxigenin (DIG)-labeled probe and visualized as red signals using the ultraView Red ISH DIG detection kit.
Estimation of EGFR and HER2 expression
HER2 expression was scored using the HercepTest scoring criterion [19]. The HER2 score was based on a scale where 0 corresponded to tumor cells that were completely negative; 1+ corresponded to faintly perceptible staining of the tumor cell membranes, 2+ corresponded to moderate staining of the entire or lateral-basal tumor cell membranes, and 3+ was strong circumferential staining of the entire or lateral-basal tumor cell membranes creating a fishnet pattern as shown in Figure S1a. The Canadian and the Dako HercepTest guidelines, which require that more than 10 % of the tumor cells be stained, were applied. Cytoplasmic staining was considered nonspecific and was not included in the scoring. As negative controls, we used normal tissues, which are expected not to express HER2, such as connective tissue areas with fibroblast cells. The expression pattern of EGFR is similar to that of HER2. The following scoring approach was used in the assessment of EGFR immunostaining [20]. Score 0 corresponded to no staining or unspecific staining of tumor cells, 1+ was weak (intensity) and incomplete membrane staining of more than 10 % of tumor cells, 2+ was moderate and complete membrane staining of more than 10 % of tumor cells, and 3+ was strong and complete membrane staining of more than 10 % of tumor cells.
For evaluation of the DISH results, black HER2 signals and red CEP17 signals were counted, and the HER2/CEP17 signal ratio was calculated. The criteria classified that a HER2/CEP17 ratio ≥2.2 was positive, a HER2/CEP17 ratio between 1.8 and 2.2 was equivocal, and a HER2/CEP17 ratio <1.8 was negative.
Statistical analysis
Differences in the incidence between the groups were analyzed with a chi-square test (or two-sided Fisher’s exact test). The level of significance was set at p < 0.05. Concordance between the primary tumor and metastasis and its estimation with kappa coefficiency was measured as described previously [21]. A kappa less than 0.4 represents poor agreement, a kappa of 0.4–0.6 indicates moderate agreement, from 0.6 to 0.8 indicates good agreement and greater than 0.8 indicates almost perfect agreement.
Results
Patient characteristics are summarized in Table 1. Briefly, as for histology, in the 85 patients with peritoneal metastases, 71 cases (84 %) were diffuse-type adenocarcinoma and 14 cases (16 %) were intestinal-type adenocarcinoma. In 52 patients with liver metastases, 34 cases (65 %) were intestinal-type adenocarcinoma and 18 cases (35 %) were diffuse-type adenocarcinoma. In patients with lymph node resection, lymph node metastases were observed in 60/62 (97 %) of the patients with peritoneal metastases and 43/48 (90 %) of the patients with liver metastases.
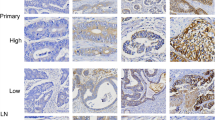
HER2 immunohistochemical staining patterns in HER2-positive gastric cancer patients with liver or peritoneal metastases are shown in Figs. 1 and 2. High and low HER2 gene amplification of HER2 (3+) and (1+) staining area in the primary tumor was representatively confirmed by DISH analysis (Fig. 1a, insets). In patients with liver metastases, there was heterogeneity of HER2 overexpression in the primary tumor to varying degrees, but in the metastatic foci, HER2 was usually overexpressed in the majority of metastatic tumors (Fig. 1a). In contrast, in most cases with peritoneal metastases, despite HER2 overexpression in the primary tumor, peritoneal metastatic foci did not always express HER2 significantly (Fig. 2b). The HER2 staining scores of the analyzed 85 patients with peritoneal metastases and 52 patients with liver metastases are shown in Table 1. The HER2 positivity (staining score 2+ and 3+) rate of the primary tumor and metastases was 61.5 and 62.5 %, respectively, in patients with liver metastases, whereas the HER2 positivity rate of the primary tumor and metastases was 22.4 and 16.4 %, respectively, in patients with peritoneal metastases (Table 2). HER2 expression of the primary tumor and liver metastases in patients with liver metastases was higher than that of peritoneal metastases. Particularly, in patients with liver metastases, the HER2-positivity rate of the intestinal type tumors, most of which were papillary adenocarcinoma, was extremely high (80.0 %) compared with that of the diffuse type (33.3 %) in the liver metastatic foci. This was not the case with primary tumors and metastases in patients with peritoneal metastases (Table 2).
HER2 and EGFR immunohistochemical staining of the primary tumor and corresponding metastases in metastatic gastric cancer patients. a HER2 protein expression of the primary tumor and liver metastases in patients with liver metastases. Note the partial and entire HER2 staining (both score 3+) in the primary and metastatic tumor, respectively. High and low HER2 gene amplifications confirmed by DISH analysis are shown in the upper and lower inset, respectively. b EGFR protein expression of the primary tumor and corresponding peritoneal metastases in patients with peritoneal metastases. Note the strong EGFR membrane staining (score 2+) in both the primary (biopsy specimen) and metastatic tumor. Bars 100 μm
Immunohistochemical staining pattern of the HER2/EGFR double-positive primary tumor and corresponding liver or peritoneal metastases in gastric cancer patients. a HER2 and EGFR expression of the primary tumor and corresponding metastasis in the patient with liver metastasis. Note that the primary tumor stained positive for both HER2 (3+) and EGFR (2+), whereas liver metastasis stained positive for HER2 (3+), but negative for EGFR (0) (right upper and lower figure is serial section). b HER2 and EGFR expression of the primary tumor and corresponding metastasis in the patient with peritoneal metastasis. The primary tumor was HER2/EGFR double positive (positive area distinct to each), whereas the peritoneal metastasis was only positive for EGFR. Bars = 100 μm
Concordance between the primary tumors and the paired metastases regarding HER2 expression was then examined. In patients with liver metastases, concordance between primary tumors and the liver metastases was 87.5 %, and the Cohen κ coefficient was 0.739, whereas concordance between primary tumors and paired metastases in patients with peritoneal metastases was 73.1 %, and the Cohen κ coefficient was 0.146 (Table 3), indicating good concordance of HER2 expression between primary tumors and the corresponding metastases only in patients with liver metastases.
EGFR immunohistochemical staining patterns in EGFR-positive gastric cancer patients with liver or peritoneal metastases are represented in Fig. 1b. EGFR expression was scored 1+, 2+ and 3+ based on the membrane staining pattern as shown in Figure S1b. In patients with peritoneal metastases, the EGFR expression in the primary tumor appeared to differ depending on the location of the stomach wall. EGFR tended to be overexpressed at the deep infiltrating area of the wall, such as the subserosal to serosal invasion area, compared with the mucosal to submucosally growing area (data not shown). The EGFR staining score for the analyzed 85 patients with peritoneal metastases and 57 patients with liver metastases is shown in Table 1. When the EGFR staining score of 1+, 2+ and 3+ is defined as EGFR positive, the EGFR positivity rate of the primary tumor and metastases was 60.0 and 70.1 %, respectively, in patients with peritoneal metastases, whereas the EGFR positivity rate of the primary tumor and metastases in patients with liver metastases was 42.3 and 37.5 %, respectively. When the staining score of 2+ and 3+ is defined as EGFR positive, the EGFR positivity rate of the primary tumor and metastases was 28.2 and 37.3 %, respectively, in patients with peritoneal metastases. The EGFR positivity rate of the peritoneal metastases (70.1 %) was higher than that of liver metastases (37.5 %) (Table 4), indicating that EGFR was preferentially expressed in the peritoneal metastatic foci in patients with peritoneal metastases.
The concordance of the EGFR positivity (score 1+/2+/3+) between the primary tumors and peritoneal metastases was 70.1 %, and the Cohen κ coefficient was 0.307 (data not shown), indicating the poor concordance between the primary tumor and peritoneal metastases. In fact, there were 11 cases with EGFR-negative primary tumors and EGFR-positive paired peritoneal metastases, and 9 cases with EGFR-positive primary tumors and EGFR-negative paired peritoneal metastases, in addition to 47 matched cases.
Coexpression of HER2 and EGFR in the primary tumor and metastases was then examined. In patients with liver metastases, the incidence of HER2/EGFR double-positive (score 2+/3+) and EGFR or HER2 single-positive metastatic tumors was in the following order: HER2+/EGFR+ (4.2 %) < HER2−/EGFR+ (12.5 %) < HER2+/EGFR− (58.3 %). A similar result was obtained in the primary tumors, indicating that HER2 single-positive tumors were predominant and HER2/EGFR double-positive tumors were rare in patients with liver metastases. In contrast, the incidence of double-positive and single-positive metastatic tumors in patients with peritoneal metastasis was in the order: HER2+/EGFR+ (4.5 %) < HER2+/EGFR− (9.0 %) < HER2−/EGFR+ (32.8 %), indicating that EGFR single-positive tumors were predominant and HER2/EGFR double-positive tumors were also rare in patients with peritoneal metastasis (Table 5). We found four cases with EGFR/HER2 double-positive primary tumors, but the EGFR- and HER2-positive areas were separate or distinct. The corresponding liver and peritoneal metastases were only positive for HER2 and EGFR, respectively (Fig. 2), indicating preferential liver (or peritoneal) metastases of the HER2 (or EGFR)-positive component in the primary tumor.
HER2 and EGFR expression in the lymph node metastasis was further investigated only in patients with liver metastasis as shown in Figure S2 and Table S1. The HER2 positivity rate was 66.6, 72.2 and 27.8 % in the primary tumor, liver metastasis and lymph node metastasis, respectively, indicating that the HER2 positivity rate of lymph node metastasis is significantly lower than that of liver metastasis (p < 0.01). In addition, concordance of the HER2 status between the primary tumor and lymph node metastasis is poor (κ coefficient = 0.129), unlike the good concordance between primary tumors and liver metastases (data not shown). On the other hand, the EGFR positivity rate was 27.8, 16.7 and 27.8 %, respectively, in the primary tumor, liver metastasis and lymph node metastasis, respectively, indicating a higher tendency in the lymph node metastasis than liver metastasis.
Discussion
In this study, we investigated the EGFR and HER2 expression in both primary tumors and metastases in gastric cancer patients with liver or peritoneal metastases by immunohistochemistry. Gene amplification analysis of HER2 and EGFR by FISH could not be carried out in this study except for several recent autopsy cases because of the invalid results due to the delay in formalin fixation (up to 3–4 days) of the old archived specimens resected between 1995 and 2000 [22, 23]. Nevertheless, we demonstrated the following interesting findings.
First, we demonstrated that HER2 was preferentially expressed in patients with liver metastases. The HER2 positivity rate of the primary and the metastatic tumor was 61.5 and 62.5 %, respectively, in patients with liver metastases, whereas the HER2 positivity rate of the primary and the metastatic tumor in patients with peritoneal metastases was 22.4 and 16.4 %, respectively. In particular, the HER2 positivity rate of the liver metastases with intestinal type histology (mostly papillary adenocarcinoma) was extremely high (80.0 %) compared with liver metastases of the diffuse type (33.3 %). Although the judgment criteria for HER2-positive or -negative in this immunohistochemical study were not identical with the HercepTest criteria including FISH analysis, the present finding suggests that HER2 overexpression can be predicted in the liver metastatic foci with papillary adenocarcinoma subtype, irrespective of the HER2 status of the primary tumor. To date, only one previous study reported the HER2 status of metastatic sites in gastric cancers [14]. That study examined various metastases sites such as the liver, pleural fluid, ascites fluid, skin, lymph nodes and peritoneum and showed a high concordance of HER2 expression between primary tumors and corresponding metastases using both IHC and FISH, but no detailed HER2-positivity rates in each metastatic site such as the liver, lymph node and peritoneum were described. In this study, we further investigated the HER2 expression of lymph node metastases in patients with liver metastases. We demonstrated a significantly lower HER2 positivity rate in lymph node metastases than liver metastases. Therefore, to our knowledge, the present study is the first to examine in detail the HER2 status of liver, lymph node and peritoneal metastases and show preferential HER2 expression in the liver metastasis. In our study, most of the HER2-positive primary tumors were partially positive, and positive cells were a rather minor component within the tumor, whereas in most of the HER2-positive liver metastases, positive cells were a major component of the metastatic foci (data not shown), suggesting preferential metastasis of the HER2-positive component within the primary tumor. The relationship between HER2 overexpression and hematogenous (liver) metastases is unknown at the present. Li et al. [24] reported that HER2 overexpression mediates a chemokine receptor, CXCR4-associated metastases. Therefore, we speculate that HER2 overexpression involves or promotes liver metastases, but not peritoneal and lymph node metastases. Further study to clarify the mechanism of preferential liver metastases using HER2-positive gastric cancer cell lines is being carried out in our laboratory [25, 26].
A second important finding is that EGFR was preferentially expressed in the metastatic foci of patients with peritoneal metastases. When EGFR positive was defined by EGFR staining scores of 1+/2+/3+, the EGFR positivity rate of the primary and metastatic tumor was 60.0 and 70.1 %, respectively, in patients with peritoneal metastases, whereas the EGFR positivity rate of the primary and metastatic tumor in patients with liver metastases was 42.3 and 37.5 %, respectively, indicating that a considerable percentage of the gastric cancer peritoneal metastases is EGFR positive. Interestingly, in the primary tumor, EGFR expression tended to increase in the invasion front deep in the stomach wall, which is a prerequisite for peritoneal metastases. Recently, Yasumoto et al. [27] reported that human gastric cancer cell lines with high peritoneal metastatic potential expressed high levels of EGFR and CXCR4. Furthermore, we previously demonstrated the significant anti-tumor and anti-peritoneal metastatic effect of cetuximab on EGFR-overexpressing gastric cancer preclinical models [28]. These findings strongly suggest that EGFR plays an important role in peritoneal metastases and that peritoneal metastases are a potential target for an anti-EGFR therapy such as cetuximab in gastric cancers.
Another interesting finding is the coexpression of HER2 and EGFR in liver and peritoneal metastasis. In lung cancer patients, synchronous overexpression of EGFR and HER2 has been reported to be a poor prognosticator [29]. We found that this was not the case for gastric cancer metastasis. In gastric cancer patients with liver metastases, HER2 single-positive tumors were predominant and HER2/EGFR double-positive tumors were rare, especially in the metastatic foci, whereas in patients with peritoneal metastases, EGFR single-positive tumors were predominant and HER2/EGFR double-positive tumors were also rare in peritoneal metastases. In four patients with HER2/EGFR double-positive primary tumors in which the HER2- and EGFR-positive areas were separate or distinct, corresponding liver metastasis was only positive for HER2 and peritoneal metastasis only positive for EGFR. These results support the previous findings that HER2 was preferentially expressed in liver metastases and EGFR preferentially expressed in peritoneal metastases and further indicate that HER2 and EGFR expressions in the metastatic sites tend to be mutually exclusive. This finding is consistent with the recent report using FISH analysis indicating that EGFR and HER2 were sometimes coamplified in the same tumor, but in mutually exclusive cells [30]. These results suggest the possibility that HER2 and EGFR overexpressions are independently associated with liver and peritoneal metastasis, respectively.
In conclusion, we immunohistochemically examined HER2 and EGFR expression in metastatic tumors from gastric cancer patients with liver metastases and patients with peritoneal metastases. It is quite difficult to collect specimens from metastatic lesions, especially in liver metastases, which is why no one has examined the HER2 and EGFR status in these metastatic sites to date. The present study is the first to demonstrate preferential expression of HER2 in liver metastases and EGFR in peritoneal metastases. Since HER2 and EGFR double-positive cases are rare, such a kind of gastric cancer metastasis is not a promising target for molecular therapy with dual anti-HER2 and anti-EGFR therapy such as lapatinib or a combination of trastuzumab with cetuximab [17]. However, the present results strongly suggest that liver metastases, especially with intestinal type histology, and peritoneal metastases are potential targets for anti-HER2 and anti-EGFR molecular therapy, respectively, in metastatic gastric cancer patients. Further clinical trials are warranted in the near future.
References
Kamangar F, Dore GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–50.
Ohtsu A. Chemotherapy for metastatic gastric cancer: past, present, and future. J Gastroenterol. 2008;43:256–64.
Sliwkowski MX, Lofgren JA, Lewis GD, Hotaling TE, Fendly BM, Fox JA, et al. Nonclinical studies addressing the mechanism of action of trastuzumab (Herceptin). Semin Oncol. 1999;26:60–70.
Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, et al. HERA study team. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36.
Guan Z, Xu B, DeSilvio ML, Shen Z, Arpornwirat W, Tong Z, et al. Randomized trial of lapatinib versus placebo added to paclitaxel in the treatment of human epidermal growth factor receptor 2-overexpressing metastatic breast cancer. J Clin Oncol. 2013;31:1947–53.
Tejpar S, Celik I, Schlichting M, Sartorius U, Bokemeyer C, Van Cutsem E. Association of KRAS G13D tumor mutations with outcome in patients with metastatic colorectal cancer treated with first-line chemotherapy with or without cetuximab. J Clin Oncol. 2012;30:3570–7.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. ToGA Trial Investigators: trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97.
Sawaki A, Ohashi Y, Omuro Y, Satoh T, Hamamoto Y, Boku N, et al. Efficacy of trastuzumab in Japanese patients with HER2-positive advanced gastric or gastrooesopageal junction cancer: a subgroup analysis of the Trastuzumab for Gastric Cancer (ToGA) study. Gastric Cancer. 2012;15:313–22.
Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490–9.
Doi T, Koizumi W, Siena S, Cascinu S, Ohtsu M, Michael H, et al. Efficacy, tolerability and pharmacokinetics of gefitinib (ZD1839) in pretreated patients with metastatic gastric cancer. Proc Am Soc Clin Oncol. 2003;22:258 (abs. 1036).
Terashima M, Kitada K, Ochiai A, Ichikawa W, Kurahashi I, Sakuramoto S, et al. ACTS-GC Group: impact of expression of human epidermal growth factor receptors EGFR and ERBB2 on survival in stage II/III gastric cancer. Clin Cancer Res. 2012;18:5992–6000.
Oh HS, Eom DW, Kang GH, Ahn YC, Lee SJ, Kim JH, et al. Prognostic implications of EGFR and HER-2 alteration assessed by immunohistochemistry and silver in situ hybridization in gastric cancer patients following curative resection. Gastric Cancer. 2013;. doi:10.1007/S10120-013-0288-0.
Carlsson J, Nordgren H, Sjöström J, Wester K, Villman K, Bengtsson NO, et al. HER2 expression in breast cancer primary tumours and corresponding metastases. Original data and literature review. Br J Cancer. 2004;90:2344–8.
Bozzetti C, Negri FV, Lagrasta CA, Crafa P, Bassano C, Tamagnini I, et al. Comparison of HER2 status in primary and paired metastatic sites of gastric carcinoma. Br J Cancer. 2011;104:1372–6.
Kim KC, Koh YW, Chang HM, Kim TH, Yook JH, Kim BS, et al. Evaluation of HER2 protein expression in gastric carcinomas: comparative analysis of 1,414 cases of whole-tissue sections and 595 cases of tissue microarrays. Ann Surg Oncol. 2011;18:2833–40.
Kataoka Y, Okabe H, Yoshizawa A, Minamiguchi S, Yoshimura K, Haga H, et al. HER2 expression and its clinicopathological features in resectable gastric cancer. Gastric Cancer. 2013;16:84–93.
Oshima Y, Tanaka H, Murakami H, Ito Y, Furuya T, Kondo E, et al. Lapatinib sensitivity of two novel trastuzumab-resistant HER2 gene-amplified gastric cancer cell lines. Gastric Cancer. 2013;. doi:10.1007/S10120-013-0290-6.
Gao FF1, Dabbs DJ, Cooper KL, Bhargava R. Bright-field HER2 dual in situ hybridization (DISH) assay vs fluorescence in situ hybridization (FISH): focused study of immunohistochemical 2+ cases. Am J Clin Pathol. 2014;141(1):102–10.
Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805.
Goldstein NS, Armin M. Epidermal growth factor receptor immunohistochemical reactivity in patients with American Joint Committee on Cancer Stage IV colon adenocarcinoma: implications for a standardized scoring system. Cancer. 2001;92:1331–46.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74.
Khoury T, Sait S, Hwang H, Chandrasekhar R, Wilding G, Tan D, et al. Delay to formalin fixation effect on breast biomarkers. Mod Pathol. 2009;22:1454–67.
Atkins D, Reiffen KA, Tegtmeier CL, Winther H, Bonato MS, Störkel S, et al. Immunohistochemical detection of EGFR in paraffin-embedded tumor tissues: variation in staining intensity due to choice of fixative and storage time of tissue sections. J Histochem Cytochem. 2004;52:893–901.
Li YM, Pan Y, Wei Y, Cheng X, Zhou BP, Tan M, et al. Up-regulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell. 2004;6:459–69.
Nakanishi H, Yasui K, Ikehara Y, Yokoyama H, Munesue S, Kodera Y, et al. Establishment and characterization of three novel human gastric cancer cell lines with differentiated intestinal phenotype derived from liver metastasis. Clin Exp Metastasis. 2005;22:137–47.
Yokoyama H, Ikehara Y, Kodera Y, Ikehara Y, Yatabe Y, Mochizuki Y, et al. Molecular basis for sensitivity and acquired resistance to gefitinib in HER2 overexpressing human gastric cancer cell lines derived from liver metastasis. Br J Cancer. 2006;95:1504–13.
Yasumoto K, Yamada T, Kawashima A, Wang W, Li Q, Donev IS, et al. The EGFR ligands amphiregulin and heparin-binding EGF-like growth factor promote peritoneal carcinomatosis in CXCR4-expressing gastric cancer. Clin Cancer Res. 2011;17:3619–30.
Hara M, Nakanishi H, Tsujimura K, Matsui M, Yatabe Y, Manabe M, et al. Interleukin-2 potentiation of cetuximab antitumor activity for epidermal growth factor receptor overexpressing gastric cancer xenografts through antibody-dependent cellular cytotoxicity. Cancer Sci. 2008;99:1471–8.
Onn A, Correa AM, Gilcrease M, Isobe T, Massarelli E, Bucana CD, et al. Synchronous overexpression of epidermal growth factor receptor and HER2-neu protein is a predictor of poor outcome in patients with stage I non-small cell lung cancer. Clin Cancer Res. 2004;10:136–43.
Tajiri R, Ooi A, Fujimura T, Dobashi Y, Oyama T, Nakamura R, et al. Intratumoral heterogeneous amplification of ERBB2 and subclonal genetic diversity in gastric cancers revealed by multiple ligation-dependent probe amplification and fluorescence in situ hybridization. Hum Pathol. 2013;. doi:10.1016/j.humpath.2013.11.004.
Acknowledgments
We thank Ms. M. Yoshimura, N. Shibata and Miss N. Saito for their expert technical assistance. This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, Culture and Technology, Japan.
Conflict of interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10120_2014_417_MOESM1_ESM.pptx
Supplementary material 1 Fig. S2 Immunohistochemical staining pattern of HER2 in the primary tumor, lymph node metastasis and liver metastases in HER2-positive gastric cancer patients with liver metastasis. a A representative case with HER2-negative lymph node metastasis. Both the primary tumor and liver metastasis are HER2 positive (3+). b A representative case with HER2-positive lymph node metastasis. HER2 is also positive in the primary tumor and liver metastasis. Bars =50 μm (PPTX 5496 kb)
10120_2014_417_MOESM3_ESM.pptx
Supplementary material 3 Fig. S1 Representative photomicrographs of HER2 and EGFR immunohistochemical staining scores in the metastatic sites. a HER2 staining scores 1+, 2+ and 3+ are indicated. b EGFR staining scores including 1+, 2+ and 3+. Bars =50 μm (PPTX 2283 kb)
Rights and permissions
About this article
Cite this article
Saito, T., Nakanishi, H., Mochizuki, Y. et al. Preferential HER2 expression in liver metastases and EGFR expression in peritoneal metastases in patients with advanced gastric cancer. Gastric Cancer 18, 711–719 (2015). https://doi.org/10.1007/s10120-014-0417-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-014-0417-4