Abstract
Background
In the preoperative evaluation for gastric cancer, high-resolution endoscopic technologies allow us to detect small accessory lesions. However, it is not known if the gastric remnant after partial gastrectomy for synchronous multiple gastric cancers has a greater risk for metachronous cancer. The purpose of this study was to determine the incidence of metachronous cancer in this patient subset compared with that after solitary cancer surgery.
Methods
Data on a consecutive series of 1,281 patients gastrectomized for early gastric cancer from 1991 to 2007 were analyzed retrospectively. The 715 gastric remnants after distal gastrectomy were periodically surveyed by endoscopic examination in Shikoku Cancer Center. Among those surveyed cases, 642 patients were pathologically diagnosed with solitary lesion (SO group) and 73 patients with synchronous multiple lesions (MU group) at the time of the initial surgery.
Results
In the follow-up period, 15 patients in the SO group and 3 patients in the MU group were diagnosed as having metachronous cancer in the gastric remnant. The cumulative 4-year incidence rate was 1.9 % in the SO group and 5.5 % in the MU group. The difference did not reach the significant level by the log-rank test.
Conclusions
The incidence of metachronous cancer is higher after multiple cancer surgery; however, the difference is not statistically significant.
Similar content being viewed by others
Introduction
Multiple gastric cancers have been known to arise in two different patterns: one is synchronous multiple cancers, and the other is metachronous secondary cancer, which develops after removal of the primary gastric cancer. When partial gastrectomy is performed for removal of the primary cancer, the metachronous cancer can be called remnant gastric cancer [1–4]. It has been reported that the incidence of metachronous gastric cancer after partial gastrectomy for early gastric cancer is 0.6–3.0 % [1, 4–6].
The incidence of synchronous multiple gastric cancers has been reported to occur in 5–8 % of surgically resected stomachs [7–11]. However, a comprehensive evaluation using serial sections of the whole stomach revealed that it was 13–15 % [10, 12], which suggests a higher incidence of latent lesions in the whole stomach [13]. If the latent lesion cannot be detected by preoperative examinations and is left in the gastric remnant, the lesion may arise as a metachronous cancer. In contrast, if the accessory lesion is located in the resected stomach, it can be pathologically diagnosed as a synchronous multiple cancer.
It has been reported that both metachronous and synchronous multiple gastric cancers are thought to derive from multicentric carcinogenesis and have similar characteristics [10, 14]. However, it is not known if the gastric remnant after partial gastrectomy for synchronous multiple cancer is at greater risk for metachronous cancer. If this is the case, an intensive postoperative endoscopy surveillance program should be implemented for this patient group to detect metachronous cancer at its early and curable stage.
There have been few reports comparing the incidence of metachronous cancer in the gastric remnant after partial gastrectomy for solitary and multiple gastric cancers [11, 15, 16]. Because these reports differ in their conclusions, it remains controversial whether it is higher after multiple cancer surgery. The purpose of this study was to clarify the incidence of metachronous cancer in the gastric remnant after synchronous multiple cancer surgery compared with solitary cancer surgery. For this study, we chose distal gastrectomy as the partial gastrectomy technique because it is the most common surgical treatment for gastric cancer [17, 18].
Patients and methods
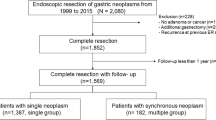
A retrospective database review of a consecutive series of 1,281 cases of gastrectomy for pathologically confirmed early gastric cancer from 1991 to 2007 in Shikoku Cancer Center identified 910 patients who underwent distal gastrectomy (Fig. 1). Negative surgical margin was confirmed by pathological examination in the resected specimens of all these patients. Following surgery, it was recommended that patients undergo surveillance endoscopic examinations at short intervals, annually, if possible, or every 2–3 years as the maximum interval. Among the afore-described patients, 715 patients underwent such endoscopic examinations in Shikoku Cancer Center with a follow-up time of more than 1 year after the surgery and were included in this study.
Selection criteria for patients in this study. A consecutive series of 1,281 gastrectomies for pathologically confirmed early gastric cancer from 1991 to 2007 was retrospectively analyzed. Of these 910 patients who underwent distal gastrectomy, 715 had periodic endoscopic surveillance in Shikoku Cancer Center and were included in this study
Early gastric cancer, defined as that invading the mucosal or submucosal layer regardless of lymph node metastasis, was classified according to the Japanese classification of gastric carcinoma [19]. The resected stomachs were processed in the usual manner. Briefly, resected stomachs were opened along the greater curvature, placed on a wooden board with the mucosa facing up, and fixed with a 10 % formalin solution for at least 24 h. Several portions, including the distal and proximal stump as well as both main and sublesions, were sliced to a thickness of 5 mm and histologically examined. For exploration of multiple lesions, resected specimens were macroscopically evaluated before and after fixation, along with preoperative evaluation, using endoscopy and upper gastrointestinal studies.
Synchronous multiple gastric cancers were defined according to the criteria reported by Moertel et al. [20]., which are as follows: (1) each lesion is histologically malignant, (2) each lesion is separated from another by the normal gastric tissue, and (3) each lesion is not the result of a local extension or metastasis of another lesion. If the depth of cancer infiltration is the same in two or more lesions, the one extending over the greatest area is regarded as the main lesion, and the other lesions are regarded as accessory lesions.
Metachronous gastric cancer was defined using the following criteria [4]: first, that curative surgery of the initial cancers had been carried out with adequate surgical margins (5 mm or more); second, that the secondary cancers were found distant from the site of the anastomosis or the suture line to exclude recurrent tumors; third, that the secondary cancers were detected by endoscopic examinations more than 1 year after the gastrectomy. For all the surveillance endoscopic examinations, careful observation was made of the mucosa of the gastric remnant. Any suspicious lesions were biopsied and examined histologically. Follow-up time was defined as the period from the gastrectomy until the detection of metachronous gastric cancer by endoscopic examination or until the last endoscopic follow-up, at which point data were censored.
Details of distal gastrectomy are described in the Japanese gastric cancer treatment guidelines [17]. The JMP 9 statistical software (SAS Institute, Cary, NC, USA) was used for all statistical analysis. The cumulative prevalence rate of metachronous gastric cancer was calculated by the Kaplan–Meier method and analyzed by the log-rank test. Pearson’s chi-square test or Wilcoxon test was used to compare the two groups. The level of significance was set at p < 0.05.
Results
Data on a consecutive series of 1,281 gastrectomized patients for early gastric cancer from 1991 to 2007 were analyzed retrospectively (Fig. 1). The 715 gastric remnants after distal gastrectomy were periodically surveyed by endoscopic examination in Shikoku Cancer Center and were included in this study. Among those surveyed cases, 642 were pathologically diagnosed with solitary lesions (SO group) and 73 with multiple lesions (MU group) at the time of the initial surgery.
The clinicopathological characteristics of the two groups at the time of the initial surgery are shown in Table 1. Patients in the MU group were significantly older and were more likely to have intestinal-type cancer upon histological examination. Although the MU group contained more male cases and more protruded tumors, these differences did not reach a significant level. The tumors of both groups were mainly located in the middle or lower third of the stomach because the patients underwent distal gastrectomy. We did not observe any significant difference in tumor size, tumor depth, node metastasis, and lymphovascular invasion between the two groups.
In the MU group, 65 patients had two lesions, 7 had three lesions, and 1 had four lesions. The median diameter of the accessory largest lesions was 7 mm (Table 2). Most accessory lesions were intramucosal and of the intestinal histological type. The accessory lesions were also located in the middle or lower third of the stomach.
The median follow-up time from gastrectomy to the last surveillance endoscopy was 50 months (range, 12–193 months) in the SO group and 50 months (range, 12–216 months) in the MU group. In the follow-up period, 15 patients in the SO group and 3 patients in the MU group were diagnosed as having metachronous cancer in the gastric remnant. The median follow-up period of the 18 patients from initial surgery to the detection of metachronous cancer was 37 months (range, 13–149 months). They underwent curative resections by remnant gastrectomy (n = 8 patients) or endoscopic mucosal resection (n = 10 patients). The reconstruction method after distal gastrectomy in this study included Billroth I anastomosis (n = 617 patients), Billroth II anastomosis (n = 19 patients), and Roux-en-Y anastomosis (n = 79 patients). Metachronous gastric cancers arose in the gastric remnant in 15 patients after Billroth I anastomosis (2.4 %), in no patient after Billroth II anastomosis (0 %), and in 3 patients after Roux-en-Y anastomosis (3.8 %). There was no statistically significant difference in the prevalence rate among these three groups.
Table 3 shows the clinicopathological characteristics of all 18 metachronous gastric cancers. Histologically, the dominant tumor type among these secondary cancers was the intestinal type. The majority of these patients had early stage T1 tumors (n = 16 patients), the remainder having T2 or T3 advanced tumors (n = 2 patients). Pathological lymph node metastasis was not found in any patients who underwent remnant gastrectomies. The cumulative 4-year incidence rate was 1.9 % in the SO group and 5.5 % in the MU group (Fig. 2). There was no significant difference between the two groups by the log-rank test (p = 0.454).
Kaplan–Meier estimates of cumulative incidence of metachronous cancer in the gastric remnant. Thin line indicates the SO (solitary lesion) group; bold line indicates the MU (synchronous multiple lesions) group. There is no significant difference between the two groups by the log-rank test (p = 0.454)
Discussion
The characteristics of synchronous multiple gastric cancers have been well studied [7, 9–12, 14, 21–23]: patients with synchronous multiple gastric cancers were more likely to be male and older and more likely to have the intestinal type of early gastric cancer. These characteristics were also observed in our current study (Table 1). With regard to tumor location, it has been known that multiple gastric cancers arise more frequently in the middle and lower than in the upper third of the stomach [9–11, 21, 23]. In addition, during whole stomach endoscopic surveillance after endoscopic mucosal resection for early cancer, the incidence rate of metachronous cancer in the upper, middle, and lower third of the stomach has been reported to be 17, 33, and 50 %, respectively [24]. These results suggest that the middle and lower third of the stomach have foci of multicentric carcinogenesis more than the upper third of the stomach. If distal gastrectomy is performed to remove the middle and lower third of the stomach, a very low incidence rate of the metachronous cancer is expected in the proximal gastric remnant. In other words, distal gastrectomy removes most of the foci of multicentric carcinogenesis from the whole stomach and reduces the incidence of metachronous cancer. We speculate that this is why the gastric remnant in the MU group failed to show a significantly greater risk for metachronous cancer in this study.
It has been well known that Helicobacter pylori infection in the gastric remnant after gastrectomy is associated with metachronous gastric cancer [25–27]. Now, Helicobacter pylori eradication is considered preventative therapy for metachronous gastric cancer [25, 28, 29]. In this study, the presence of Helicobacter pylori infection was confirmed at the time of the first surgery in 13 of 18 patients who developed metachronous gastric cancer. Because none of the patients received Helicobacter pylori eradication therapy after the first surgery, the infection remained in the remnant stomach in 10 of the 13 patients at the time of the second treatment. Therefore, among the metachronous gastric cancer subset, the Helicobacter pylori infection rate was 72 % at the time of the first surgery and 56 % in the gastric remnant. Because the median age of this patient group was 70 years, these infection rates were not very high [22, 30, 31]. However, Helicobacter pylori infection in this subset may be associated with the incidence of metachronous cancer.
It has been reported that Billroth II anastomosis is associated with gastric remnant cancer after distal gastrectomy for peptic ulcer because of duodenogastric reflux [32, 33]. In this study, the incidence rates of metachronous gastric cancers after Billroth I anastomosis, Billroth II anastomosis, and Roux-en-Y anastomosis were 2.4, 0, and 3.8 %, respectively. The incidence rate after Billroth II anastomosis was even lower than the others. We speculate that this is because the duodenogastric reflux after Billroth II anastomosis induces the primary gastric remnant cancer in the long term after benign gastric ulcer surgery, but does not induce secondary metachronous cancer in the gastric remnant after early gastric cancer surgery [33].
Fujita et al. [11] have reported that the gastric remnant after synchronous multiple cancer surgery has a higher risk of metachronous cancer. They also have reported that a combination of diffuse-type synchronous multiple cancers at the time of the initial surgery was a potential risk factor for metachronous cancer in the gastric remnant [34]. However, in our current study, all three patients in the multiple group who developed metachronous cancer had a combination of intestinal-type synchronous multiple cancers at the time of the initial surgery. Limitations of this study are the relatively small number of patients and the relatively few events in the MU group. Therefore, we need to increase the study size to further clarify a risk factor for metachronous cancer in future.
We have previously reported that male gender, elder age, submucosal invasion, and proximal gastrectomy at the time of the first surgery were independent risk factors for the metachronous cancer after early cancer surgery [4]. In that report, we recommended yearly or biyearly surveillance endoscopy for patients with any of these risk factors and every 3 years in patients with no risk factors to detect metachronous cancer at its curable stage. Because the gastric remnant after distal gastrectomy for synchronous multiple cancers did not show significantly higher risk for metachronous cancer than that seen after solitary cancer surgery in this study, we think this patient subset can follow our previous recommendations. In conclusion, the incidence of metachronous cancer in the gastric remnant is higher after multiple cancer surgery; however, the difference is not statistically significant.
References
Ikeda Y, Saku M, Kishihara F, Maehara Y. Effective follow-up for recurrence or a second primary cancer in patients with early gastric cancer. Br J Surg. 2005;92:235–9.
Furukawa H, Iwanaga T, Hiratsuka M, Imaoka S, Ishikawa O, Kabuto T, et al. Gastric remnant cancer as a metachronous multiple lesion. Br J Surg. 1993;80:54–6.
Nozaki I, Kurita A, Nasu J, Kubo Y, Aogi K, Tanada M, et al. Higher incidence of gastric remnant cancer after proximal than distal gastrectomy. Hepatogastroenterology. 2007;54:1604–8.
Nozaki I, Nasu J, Kubo Y, Tanada M, Nishimura R, Kurita A. Risk factors for metachronous gastric cancer in the remnant stomach after early cancer surgery. World J Surg. 2010;34:1548–54.
Onodera H, Tokunaga A, Yoshiyuki T, Kiyama T, Kato S, Matsukura N, et al. Surgical outcome of 483 patients with early gastric cancer: prognosis, postoperative morbidity and mortality, and gastric remnant cancer. Hepatogastroenterology. 2004;51:82–5.
Hosokawa O, Kaizaki Y, Watanabe K, Hattori M, Douden K, Hayashi H, et al. Endoscopic surveillance for gastric remnant cancer after early cancer surgery. Endoscopy. 2002;34:469–73.
Yamagiwa H, Yoshimura H, Matsuzaki O, Ishihara A. Pathological study of multiple gastric carcinoma. Acta Pathol Jpn. 1980;30:421–6.
Kim DY, Joo JK, Ryu SY, Park YK, Kim YJ, Kim SK. Clinicopathologic characteristics of gastric carcinoma in elderly patients: a comparison with young patients. World J Gastroenterol. 2005;11:22–6.
Kodama M, Tur GE, Shiozawa N, Koyama K. Clinicopathological features of multiple primary gastric carcinoma. J Surg Oncol. 1996;62:57–61.
Kosaka T, Miwa K, Yonemura Y, Urade M, Ishida T, Takegawa S, et al. A clinicopathologic study on multiple gastric cancers with special reference to distal gastrectomy. Cancer (Phila). 1990;65:2602–5.
Fujita T, Gotohda N, Takahashi S, Nakagohri T, Konishi M, Kinoshita T. Clinical and histopathological features of remnant gastric cancers, after gastrectomy for synchronous multiple gastric cancers. J Surg Oncol. 2009;100:466–71.
Esaki Y, Hirokawa K, Yamashiro M. Multiple gastric cancers in the aged with special reference to intramucosal cancers. Cancer (Phila). 1987;59:560–5.
Lee HL, Eun CS, Lee OY, Han DS, Yoon BC, Choi HS, et al. When do we miss synchronous gastric neoplasms with endoscopy? Gastrointest Endosc. 2010;71:1159–65.
Honmyo U, Misumi A, Murakami A, Haga Y, Akagi M. Clinicopathological analysis of synchronous multiple gastric carcinoma. Eur J Surg Oncol. 1989;15:316–21.
Yanadori E, Oguma H, Sasagawa T, Kitamura Y, Takasaki K. Clinicopathological study of multifocal gastric cancer. Jpn J Gastroenterol Surg. 2001;34:9–14 (in Japanese).
Kodera Y, Yamamura Y, Torii A, Uesaka K, Hirai T, Yasui K, et al. Considerations on the management of multiple gastric cancer from the viewpoint of postoperative surveillance and diagnosis of remnant cancer. Jpn J Gastroenterol Surg. 1995;28:2092–6 (in Japanese).
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113–23.
Maruyama K, Kaminishi M, Hayashi K, Isobe Y, Honda I, Katai H, et al. Gastric cancer treated in 1991 in Japan: data analysis of nationwide registry. Gastric Cancer. 2006;9:51–66.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma—2nd English edition. Gastric Cancer. 1998;1:10–24.
Moertel CG, Bargen JA, Soule EH. Multiple gastric cancers: review of the literature and study of 42 cases. Gastroenterology. 1957;32:1095–103.
Nitta T, Egashira Y, Akutagawa H, Edagawa G, Kurisu Y, Nomura E, et al. Study of clinicopathological factors associated with the occurrence of synchronous multiple gastric carcinomas. Gastric Cancer. 2009;12:23–30.
Lee IS, Park YS, Kim KC, Kim TH, Kim HS, Choi KD, et al. Multiple synchronous early gastric cancers: high-risk group and proper management. Surg Oncol. 2012;21:269–73.
Peng J, Wang Y. Epidemiology, pathology and clinical management of multiple gastric cancers: a mini-review. Surg Oncol. 2010;19:e110–4.
Nasu J, Doi T, Endo H, Nishina T, Hirasaki S, Hyodo I. Characteristics of metachronous multiple early gastric cancers after endoscopic mucosal resection. Endoscopy. 2005;37:990–3.
Matsukura N, Tajiri T, Kato S, Togashi A, Masuda G, Fujita I, et al. Helicobacter pylori eradication therapy for the remnant stomach after gastrectomy. Gastric Cancer. 2003;6:100–7.
Nakagawara H, Miwa K, Nakamura S, Hattori T. Duodenogastric reflux sustains Helicobacter pylori infection in the gastric stump. Scand J Gastroenterol. 2003;38:931–7.
Onoda N, Maeda K, Sawada T, Wakasa K, Arakawa T, Chung KH. Prevalence of Helicobacter pylori infection in gastric remnant after distal gastrectomy for primary gastric cancer. Gastric Cancer. 2001;4:87–92.
Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392–7.
Shiotani A, Uedo N, Iishi H, Yoshiyuki Y, Ishii M, Manabe N, et al. Predictive factors for metachronous gastric cancer in high-risk patients after successful Helicobacter pylori eradication. Digestion. 2008;78:113–9.
Matsuo T, Ito M, Takata S, Tanaka S, Yoshihara M, Chayama K. Low prevalence of Helicobacter pylori-negative gastric cancer among Japanese. Helicobacter. 2011;16:415–9.
Asaka M, Kimura T, Kudo M, Takeda H, Mitani S, Miyazaki T, et al. Relationship of Helicobacter pylori to serum pepsinogens in an asymptomatic Japanese population. Gastroenterology. 1992;102:760–6.
Domellof L. Gastric carcinoma promoted by alkaline reflux gastritis—with special reference to bile and other surfactants as promoters of postoperative gastric cancer. Med Hypotheses. 1979;5:463–76.
Tersmette AC, Offerhaus GJ, Tersmette KW, Giardiello FM, Moore GW, Tytgat GN, et al. Meta-analysis of the risk of gastric stump cancer: detection of high risk patient subsets for stomach cancer after remote partial gastrectomy for benign conditions. Cancer Res. 1990;50:6486–9.
Fujita T, Gotohda N, Takahashi S, Nakagohri T, Konishi M, Kinoshita T. Relationship between the histological type of initial lesions and the risk for the development of remnant gastric cancers after gastrectomy for synchronous multiple gastric cancers. World J Surg. 2010;34:296–302.
Acknowledgments
This work was supported in part by the National Cancer Center Research and Development Fund (23-A-19).
Conflict of interest
This article has no potential or real conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nozaki, I., Hato, S., Kobatake, T. et al. Incidence of metachronous gastric cancer in the remnant stomach after synchronous multiple cancer surgery. Gastric Cancer 17, 61–66 (2014). https://doi.org/10.1007/s10120-013-0261-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-013-0261-y