Abstract
Background
A submucosal tumor (SMT) of the stomach, which is an occasional finding during routine upper gastrointestinal endoscopy, may pose diagnostic and therapeutic challenges.
Methods
To assess whether endoscopic submucosal dissection (ESD) is a feasible approach to definitively cure SMTs, the authors performed a retrospective cohort study with two endoscopic italian centers.
Results
The study consisted of 20 patients with SMTs who underwent ESD. The patients underwent ESD and were followed up by endoscopy. We analyzed complete resection rate, frequency of complications, and survival. The overall rate of R0 resection was 90 % (18/20), with two endoscopic failures, one for a submucosal tumor and one for a neoplasm deeply infiltrating the proper muscle layer. The median procedure time was 119.1 min (range 40–240 min). The median size of the resected specimens was 29 mm (range 15–60 mm). Perforation occurred in 3 patients; all were treated conservatively. There were no cases of severe bleeding. Based on histopathological findings, 6 cases of ectopic pancreas, 1 of ectopic spleen, 3 of leiomyoma, and 10 of gastrointestinal stromal tumor (GIST) were diagnosed. Complete resection was obtained in all GIST cases. Among the 10 GIST cases treated by ESD, no death occurred: the 5-year disease-specific survival rate was 100 %.
Conclusions
The high success rate of 90 % and the low incidence of complications should indicate ESD is the correct diagnostic and definitive treatment in selected patients.
Similar content being viewed by others
Introduction
Submucosal tumors (SMTs) of the stomach are occasional findings during routine upper gastrointestinal endoscopy [1]. They may arise from any of the layers of the intestinal wall and are classified as nonepithelial, mesenchymal neoplasms [2]. The most common type is the gastrointestinal stromal tumor (GIST), first described by Mazur and Clark in 1983 [3], which originates from the interstitial cells of Cajal. These cells have myogenic and neurogenic architecture and are found within the mesenteric plexus, submucosa, and muscularis propria of the gastrointestinal tract. Initially considered to be of negligible importance, the malignant potential of GISTs, through mutations of the c-kit or PDGFR-α, proto-oncogenes, is now recognized among all the SMTs [4, 5]. GIST are commonly found in the stomach (40–70 %), small intestine (20–40 %), and colon and rectum (5–15 %). On the basis of these findings, their histological characterization is of paramount importance to determine proper treatment. Endoscopic ultrasonography (EUS) can provide information about the size, layer of origin, margins, and echogenicity of SMTs, but multiple biopsies, either through conventional endoscopy or EUS guided, often fail to distinguish between benign and malignant disease [6]. The treatment of choice has, until now, consisted of surgical resection with an open or laparoscopic approach [7]. However, this technique is overly invasive for lesions with a diameter <2 cm and with a low mitotic count (<5) as determined by the examination of microscopic high-power fields (HPFs) [8]. A new endoscopic mini-invasive technique, endoscopic submucosal dissection (ESD), enables the resection of epithelial lesions of any diameter “en bloc.” ESD is gaining broad acceptance for the treatment of early neoplastic lesions of the stomach and has been proposed for the treatment of SMTs [9, 10]. In this study we present the results of our experience in the use of ESD to treat patients with SMTs. The main objective of the study was to evaluate the rate of complete resection and complication of ESD.
Patients and methods
This study was a retrospective analysis encompassing the period between May 2005 and May 2011. During that time, ESD was used to treat 20 patients with gastric SMTs who underwent endoscopy at the Emergency Unit of Surgical Endoscopy, General Hospital of Verona or at the Operative Units of Endoscopy, USI Group Rome. These 20 patients comprised the study population. The mean age of the 7 men (35 %) and 13 women (65 %) was 57.1 years (range 23–83 years). The data considered herein were collected from clinical records. Patients were submitted to ESD in cases of tumor with diameter between 1 and 6 cm, whereas patients with an evident extraluminal growth assessed by EUS or with an echopattern consistent with lipoma were excluded by the endoscopic treatment. Endoscopic mucosal resection was suggested to patients with a tumor up to 1 cm; patients with a tumor more than 6 cm in diameter were referred for surgery.
Endoscopic procedure
Upper endoscopy was performed in all patients with the aim of evaluating the degree of elevation of the mass and its angle with respect to the gastric mucosa (acute or obtuse). EUS was performed to determine the size, margins, echopattern, and histological layer of origin of the lesion, and EUS-fine needle aspiration (FNA) was performed for lesions with a diameter of more than 20 mm (8 cases) because it was considered useful only in this clinical setting. A computed tomography (CT) scan was performed for all patients.
ESD according to Hosokawa et al. [11] was performed as follows: SMTs with a degree of elevation >50 % and with an acute angle with respect to the underlying mucosa were endoscopically treated through a hemicircumferential incision around the lesion. For tumors of low elevation and at an obtuse angle with respect to the underlying mucosa, a complete circumferential incision was performed to make easier the spontaneous spillage of the lesions from the deeper layer of the gastric wall (Fig. 1). Both an insulated-tip (IT) knife (Olympus, Tokyo, Japan) and a hook knife (Olympus) were used to achieve submucosal dissection. The right-angled 1-mm tip of the hook knife was used in the debridement of the tumor from the muscle layer. The specimens were retrieved using a Multi Bag (Endo-Technik, Germany). All procedures were performed with the patients under general anesthesia. Evident perforation of the gastric wall was treated with endoscopic clipping. The endoscopic treatment was considered complete when the lesion was resected en bloc and when no residual tumor was observable on the resected wound. Histopathological evaluation was based on the examination of HPFs, focusing on cell type and mitotic count. Immunohistochemical analysis was directed at the markers c-kit (cd 117), cd 34, smooth muscle actin (sma), and s-100.
All patients provided informed consent for ESD, opting for the endoscopic treatment instead of simple follow-up or the surgical option.
Follow-up
All patients were followed up by conventional endoscopy 2 and 6 months after the procedure and once a year for 5 years. CT scan examination has been provided once a year for 5 years.
Ethics
The study was approved by the Ethics Committees of the respective institutions.
Statistics
The sample size was not formally evaluated, but based on the sample size of our study the 95 % confidence interval for an 80 % rate of complete resection is ~50–99 %. Descriptive statistics (mean, SD, proportion, range) were computed. Differences between means were compared by the two-tailed Student’s t test. The software SPSS ver. 14 was used. Survival was computed using actuarial methods.
Results
In 3 of the 20 patients, the clinical symptoms at presentation of the cancer consisted of gastrointestinal bleeding whereas the remaining 17 patients had no remarkable onset of symptoms.
The CT scans of all the patients were negative. In 2 patients, EUS failed in the correct evaluation of the tumor in the gastric wall: in one submucosal tumor, an overlapping to the muscularis propria was diagnosed, while in another case no extension of the tumor to the proper muscle layer was confirmed at the end of the endoscopic procedure. FNA was performed in 8 of the tumors with only 2 of 6 cases confirmed as GIST (33.3 %). On the basis of EUS findings, definitive diagnosis was achieved in a few cases. At endoscopy, in 13 patients (65 %) the grade of protrusion of the tumor was >50 %, thus forming an acute angle with respect to the gastric mucosa. Table 1 gives the details of the 20 patients evaluated. The median procedure time was 119.1 min (range 40–240). The median size of the resected specimen was 29 mm. The largest specimen, which measured 60 × 25 mm, was located in the gastric body; it had an hard elastic consistency and an elongated shape that facilitated its retrieval after the resection in two pieces by a conventional snare. Intraoperative perforations occurred in 3 patients. The SMTs of these patients were those with the largest diameters among our series (40, 50, and 60 mm). In 1 case, perforation occurred at the end of the procedure; the patient was treated conservatively. In the other 2 cases, the perforation occurred during the circumferential incision: the defects were closed with two endoclips and the procedure was completed normally. No case of severe bleeding occurred in our series (Table 2; Figs. 2, 3, 4). ESD was completed successfully in 18 of the 20 patients, corresponding to a success rate of 90 % (lower 95 % confidence interval, 55.6). All the lesions confined to the submucosal layer were completely treated by ESD, except one in which the procedure was stopped because of tight adhesions of the tumor to the muscle layer; the patient was submitted to a combined endoscopically assisted laparoscopic approach. In 3 cases of our series the tumors were confined to the proper muscle layer, but the procedure was successful in 2 cases with only 1 patient converted to open surgery.
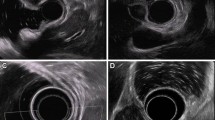
a Submucosal tumor 15 mm in diameter, at the angulus, lesser curvature of the gastric body with a protrusion more than 50 % and acute angle. b Endoscopic ultrasonography shows the submucosal layer origin of the tumor. c Endoscopic dissection of the submucosal layer beneath the tumor under direct vision with insulated-tip (IT) knife. d Complete dissection of the tumor. e Histological examination revealed a gastrointestinal stromal tumor (GIST) with spindle cells highly positive at CKIT/CD117. f Histological view with hematoxylin and eosin (H&E) stain. e ×40; f ×20
a Submucosal tumor 60 mm in maximum diameter (elongated shape), at the gastric fundus, lesser curvature with a protrusion more than 50 % and acute angle. b Endoscopic ultrasonography shows the submucosal layer origin of the tumor. c A hemicircumferential incision and complete dissection is obtained with the IT knife. d Endoscopic finding of ulcer bed after endoscopic submucosal dissection (ESD). e Histological examination revealed a GIST tumor with epithelioid cells, positive at CKIT/CD117. f Histological view with H&E stain. e ×40; f ×20
Two cases of submucosal tumors with different protrusion from the gastric wall and their implication in the modality of endoscopic resection. a Submucosal tumor of the upper body close to the cardias with a protrusion more than 50 % and acute angle. b A hemicircumferential incision is sufficient to resect the tumor. c Submucosal tumor of the gastric antrum with a protrusion less than 50 % and obtuse angle. d A circumferential incision is performed to dissect the tumor
Based on the histopathological findings, six cases of ectopic pancreas, one of ectopic spleen, three cases of leiomyoma, and ten of GIST were diagnosed. Complete resection was defined as en bloc resection of the lesion with the entire capsule preserved and a small amount of normal tissue visible at the deep margin of the lesion; this was obtained in all the cases. Among the ten patients with GISTs, the mean (SD) tumor size was 28 (range 1–36 mm). Low HPF mitotic counts (<5 mitotic figures/50 HPF) were determined in nine of the GISTs and >5 were found in the remaining GIST. The latter occurred in an ASA IV patient with a 6-cm lesion in whom surgical treatment was considered high risk.
Survival
Among the ten GIST patients, no death occurred. One death occurred for another cause (hepatic cirrhosis); thus, the 5-year survival rate was 100 %.
Discussion
Although rare, SMTs of the stomach are a diagnostic as well as a therapeutic challenge. In these tumors, the diagnostic accuracy of EUS is high in terms of detecting the layer of origin and the size of the lesion, but this technique is unable to differentiate subepithelial from mesenchymal tumors and benign from malignant lesions, as confirmed in our series [12]. Instead, a correct diagnosis is possible only with histology, performed on specimens collected during ESD or surgery. Eight EUS fine-needle aspiration (FNA) studies in which a total of 426 gastric tumors were examined reported a diagnostic yield of 63 % for histology, similar to that of EUS-TCB (Trucut needle biopsy) [13]. However, the mucosal specimens obtained using either of these procedures are usually too small for an accurate histological examination [14]. In our series, EUS failed to establish the layer of origin of the lesion in two patients. In one the tumor was compressed by the proper muscle layer, mimicking its infiltration; in the other, EUS showed only the submucosal involvement of the tumor while endoscopic dissection demonstrated an infiltration over the proper muscle layer, such that open surgery was required. FNA in our series was able to confirm GIST only in 2 of 8 cases without the evaluation of the mitotic index. The high success rate (90 % in our series) achieved with ESD and the absence of relevant complications suggest this procedure as a diagnostic and in some case therapeutic option in the resection of SMTs [15–19]. The main limitation of this study was that patients were assigned to ESD based solely on the patient’s attitude. Despite this limitation, our study provides information and identifies the potential complications associated with ESD. Compared to Hosokawa’s original description [11], our ESD procedure involves several slight modifications that, in our opinion, make it easier to perform. We considered a complete resection of GIST to have occurred when severe histological criteria were respected (en bloc resection; entire capsule preserved and normal tissue visible at the deepest margin of the lesion). The follow-up provided no evidence of recurrence in the nine surviving patients with GIST. Histological evaluation of the resected GIST specimens showed only one high-grade tumor, in an ASA IV patient in whom a lesion of 6 cm was detected. The patient underwent ESD because the risk associated with surgical treatment was considered to be too high. In the remaining patients with GIST, the results were consistent with a lower percentage of malignancy. Another mini-invasive procedure, named laparoscopic endoscopic cooperating surgery, a type of endoscopic procedure assisted by a laparoscopic approach, has been proposed for the treatment of these lesions [20–23]. Hiki et al. refer to seven patients all successfully treated with this procedure. The lesions were located close to the esophagogastric junction or in the posterior wall of the stomach, and three of the lesions had a diameter of more than 5 cm, so this technique seems suitable in case of lesions with a difficult location and with larger diameter that are not adequate for the endoscopic treatment [24]. Therefore, ESD could be an acceptable alternative to another modality of treatment for those lesions with concerning EUS features (no extraluminal growth or absence of echopattern consistent with lipoma).
In our experience, ESD resulted in a high success rate of 90 % and a low incidence of complications, with three perforations, limited to patients with the largest lesions and all treated conservatively. Thus, in cases in which the available endoscopic techniques such as EUS do not provide a definitive diagnosis, ESD offers a reliable alternative, allowing definitive treatment of SMTs and GISTs. In case of tumors limited to submucosal or with a minimum invasion of the proper muscle layer and in absence of deep central ulceration, endoscopic treatment can be considered after careful evaluation of each single patient [25–27]. Our experience is, however, limited to a few patients and our findings remain to be confirmed in a larger series.
References
Hwang JH, Kimmey MB. The incidental upper gastrointestinal subepithelial mass. Gastroenterology. 2004;126:301–7.
Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumors: recent advances in understanding of their biology. Hum Pathol. 1999;30:1213–20.
Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol. 1983;7:507–19.
Evans HL. Smooth muscle tumors of the gastrointestinal tract. A study of 56 cases followed for a minimum of 10 years. Cancer (Phila). 1985;56:2242–50.
Langer C, Gunawan B, Schuler P, Huber W, Fuzesi L, Becker H. Prognostic factors influencing surgical management and outcome of gastrointestinal stromal tumours. Br J Surg. 2003;90:332–9.
Lachter J, Bishara N, Rahimi E, Shiller M, Cohen H, Reshef R. EUS clarifies the natural history and ideal management of GISTs. Hepatogastroenterology. 2008;55:1653–6.
Wilhelm D, Delius S, Burian M, Schneider A, Frimberger E, Meining A, Feussner H. Simultaneous use of laparoscopy and endoscopy for minimally invasive resection of gastric subepithelial masses—analysis of 93 interventions. World J Surg. 2008;32:1021–8.
Connolly EM, Gaffney E, Reynolds JV. Gastrointestinal stromal tumours. Br J Surg. 2003;90:1178–86.
Katoh T, Itoh Y, Mohri T, Suzuki H. Endoscopic enucleation of gastrointestinal stromal tumors of the stomach: report of five cases. World J Gastroenterol. 2008;14:2609–11.
Bialek A, Wiechowska-Kozlowska A, Pertklewicz J, Polkowski M, Milkiewicz P, Karpinska K, Lawniczak M, Starzynska T. Endoscopic submucosal dissection for treatment of gastric subepithelial tumors (with video). Gastrointest Endosc. 2012;75:276–86.
Ohkuwa M, Hosokawa K, Boku N, Ohtu A, Tajiri H, Yoshida S. New endoscopic treatment for intramucosal gastric tumours using an insulated-tip diathermic knife. Endoscopy. 2001;33:221–6.
Palazzo L, Landi B, Cellier C, Cuillerier E, Roseau G, Barbier JP. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumours. Gut. 2000;46:88–92.
Ha CY, Shah R, Chen J, Azar RR, Edmundowicz SA, Early DS. Diagnosis and management of GI stromal tumors by EUS-FNA: a survey of opinions and practices of endosonographers. Gastrointest Endosc. 2009;6:1039–44.
Waterman AL, Grobmyer SR, Cance WG, Hochwald SN. Is endoscopic resection of gastric gastrointestinal stromal tumors safe? Am Surg. 2008;12:1186–9.
Von Renteln D, Riecken B, Walz B, Muehleisen H, Caca K. Endoscopic GIST resection using FlushKnife ESD and subsequent perforation closure by means of endoscopic full-thickness suturing. Endoscopy. 2008;40(suppl 2):E224–5.
Rösch T, Sarbia M, Schumacher B, Deinert K, Frimberger E, Toermer T, Stolte M, Neuhaus H. Attempted endoscopic en bloc resection of mucosal and submucosal tumors using insulated-tip knives: a pilot series. Endoscopy. 2004;9:788–801.
Lee IL, Lin PY, Tung SY, Shen CH, Wei KL, Wu CS. Endoscopic submucosal dissection for the treatment of intraluminal gastric subepithelial tumors originating from the muscularis propria layer. Endoscopy. 2006;38:1024–8.
Park YS, Park SW, Kim T, Song Young S, Choi EH, Bock Chung J, Kang Kyung J. Endoscopic enucleation of upper-GI submucosal tumors by using an insulated-tip electrosurgical knife. Gastrointest Endosc. 2006;59:409–15.
Sun S, Ge N, Wang C, Lu Q. Endoscopic band ligation of small gastric stromal tumours and follow-up by endoscopic ultrasonography. Surg Endosc. 2007;21:574–8.
Sakamoto Y, Sakaguchi Y, Akimoto H et al. Safe laparoscopic resection of a gastric gastrointestinal stromal tumor close to the esophagogastric junction. Surg Today. 2012;42:708–11.
Marano L, Torelli F, Schettino M, et al. Combined laparoscopic-endoscopic “rendez-vous” procedure for minimally invasive resection of gastrointestinal stromal tumors of the stomach. Am Surg. 2011;77:1100–2.
Privette A, McCahill L, Borrazzo E, Single RM, Zubarik R. Laparoscopic approaches to resection of suspected gastric gastrointestinal stromal tumors based on tumor location. Surg Endosc. 2008;22:487–94.
Sasaki A, Koeda K, Obuchi T, Nakajima J, Nishizuka S, Terashima M, Wakabayashi G. Tailored laparoscopic resection for suspected gastric gastrointestinal stromal tumors. Surgery (St. Louis). 2010;147:516–20.
Hiki N, Yamamoto Y, Fukunaga T, Yamaguchi T, Nunobe S, Tokunaga M, Miki A, Ohyama S, Seto Y. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc. 2008;22:1729–35.
Hoteya S, Iizuka T, Kikuchi D, Yahagi N. Endoscopic submucosal dissection for gastric submucosal tumor, endoscopic sub-tumoral dissection. Dig Endosc. 2009;21:266–9.
Hwang JC, Kim JH, Kim JH. Endoscopic resection for the treatment of gastric subepithelial tumors originated from the muscularis propria layer. Hepatogastroenterology. 2009;56:1281–6.
Probst A, Golger D, Arnholdt H. Endoscopic submucosal dissection of early cancers, flat adenomas and submucosal tumors in the gastrointestinal tract. Clin Gastroenterol Hepatol. 2009;7:149–55.
Conflict of interest
Dr. Filippo Catalano, Dr. Luca Rodella, Dr. Francesco Lombardo, Prof. Marco Silano, Dr. Anna Tomezzoli, Dr. Arnaldo Fuini, Dr. Maria Antonietta Di Cosmo, Prof. Giovanni de Manzoni, and Dr. Antonello Trecca have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MOV 62439 kb)
Rights and permissions
About this article
Cite this article
Catalano, F., Rodella, L., Lombardo, F. et al. Endoscopic submucosal dissection in the treatment of gastric submucosal tumors: results from a retrospective cohort study. Gastric Cancer 16, 563–570 (2013). https://doi.org/10.1007/s10120-012-0225-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-012-0225-7