Abstract
Background
Patient-controlled epidural analgesia (PCEA) has not been widely used after gastrectomy, although, in other abdominal surgery, it benefits patients more than patient-controlled intravenous analgesia (PCIA). We attempted to determine the effect of PCEA compared with PCIA on postoperative pain control and recovery after gastrectomy for gastric cancer.
Methods
A randomized controlled clinical trial that included patients undergoing D2 radical gastrectomy for gastric cancer was conducted for this study. Patients were randomized to a morphine–bupivacaine PCEA group and a morphine PCIA group. Postoperative outcomes such as pain, fasting blood glucose (FBG), time to first passage of flatus, complications, and time staying in hospital after surgery were compared with an intention-to-treat analysis.
Results
Between March 2010 and October 2010, 67 patients were randomized and 60 were evaluated. The PCEA group showed lower pain scores both at rest and on coughing after the operation (P < 0.05). FBG after the operation was significantly lower in the PCEA group than that in the PCIA group (P < 0.05). Time to first passage of flatus after surgery was shorter in the PCEA group (P < 0.05), while there were no significant differences regarding the incidence of complications between the two groups in terms of the clinical records. The length of hospital stay in the PCEA group was 10.7 ± 1.7 days, which was significantly shorter than that in the PCIA group (11.9 ± 1.8 days, P < 0.05).
Conclusions
After gastrectomy for gastric cancer, PCEA, compared with PCIA, offered safer pain relief with superior pain control and resulted in a lower stress response and a quicker return of bowel activity.
Similar content being viewed by others
Introduction
Gastric cancer is one of the most common malignant tumors of the digestive system in China [1] and surgery with distal gastrectomy or total gastrectomy is the most effective but invasive treatment for this cancer. Although the surgical techniques have been greatly improved, the recovery from such invasive operations remains a prominent clinical problem. Poor pain control, metabolic and endocrine responses to stress [2], delayed return of bowel function [3], and other complications caused by the surgery can all lead to delays in postoperative recovery, resulting in long stays in hospital and increased costs.
Both patient-controlled epidural analgesia (PCEA) and patient-controlled intravenous analgesia (PCIA) provide effective postoperative analgesia and both have been widely used in the past few decades. Some researchers have pointed out that PCEA showed more advantages in postoperative analgesia and could relieve pain both at rest and on coughing more effective than PCIA after some gynecological and thoracic operations [4, 5]. Besides, PCEA can attenuate postoperative stress, and offer a rapid return of bowel activity after operations [6]. All these advantages may help to accelerate recovery after an operation and shorten the length of stay in hospital.
The routine use of PCEA after operations for gastric cancer has not been universally incorporated into clinical practice, even though there has been evidence that it benefited patients after some other abdominal surgeries. That PCEA is not routinely used after gastric cancer surgery is likely due to the lack of published data on the use of perioperative regional anesthesia and analgesia in this type of surgery. We are currently unaware of any published prospective randomized trials comparing PCEA and PCIA in patients who have undergone radical resection for gastric cancer. The role of PCEA (using a combination of a local anesthetic and an opioid) on postoperative recovery parameters such as pain, nausea, vomiting, return of bowel function, and length of hospital stay has not been determined. Therefore, we performed a prospective randomized study in patients undergoing gastrectomy for gastric cancer, comparing bupivacaine–morphine PCEA and morphine PCIA in terms of postoperative pain relief, fasting blood sugar levels, first time to flatus, complications, and length of hospital stay after the surgery, and we also evaluated the use of PCEA in these patients.
Patients, materials, and methods
The study was carried out in accordance with the Helsinki Declaration and the guidelines published by the CONSORT group; prior approval of the research project was granted by the ethics committees of the Second Military Medical University and Changzheng Hospital, Shanghai, China. Before participation, patients received information about the study, including details of the treatment procedure, and gave their written consent. Patients undergoing D2 radical gastrectomy for gastric cancer were eligible for inclusion, and were randomly assigned to either the PCEA or PCIA group. A D2 radical gastrectomy was defined as gastrectomy with systematic dissection of lymph nodes in the second tier [7] and with clear histological margins. Patients in the PCEA group received PCEA, while patients in the PCIA group received PCIA. Exclusion criteria included age younger than 20 years or older than 75, diabetes mellitus or impaired glucose tolerance, medication that could potentially affect the outcome (such as pain control, fasting blood glucose, return of bowel activity and others), insulin sensitivity, urinary system disease, having received preoperative therapy, having symptoms of obstruction, allergy to amide local anesthetics or opioids, coexisting diseases that could affect the reliability of clinical assessments, known or suspected drug abuse, and pregnancy.
In both groups of patients, a gastric tube and a urinary drainage tube were inserted before surgery. Patients in the PCEA group had one catheter inserted at the T8–9 level before the induction of general anesthesia. In these patients, postoperative analgesia was provided by the epidural infusion of 0.05 % bupivacaine and 100 μg/mL morphine at the basal rate of 4 mL/h for 48 h, supplemented by rescue boluses of 4 mL, with a 30-min lock-out period, using an electronic patient-controlled analgesia (PCA) pump. Each participant in the PCIA group received intravenous morphine at a continuous basal rate of 1 mg/h, with morphine rescue boluses of 1 mg every 10 min if needed, controlled with a PCA pump. If a patient complained of inadequate pain relief, pethidine was used as a supplemental drug for breakthrough pain in both groups. The gastric tube was removed after the passage of flatus without nausea, vomiting, or other symptoms of obstruction. The urinary drainage tube was removed 72 h after the operation. Urinary retention was defined as the need for catheterization. Patients receiving catheterization included those who had not voided for 6 h after the removal of the urinary drainage tube, plus those who had one of the following characteristics: (a) patients who had an urge to void but could not void and (b) patients whose bladder ultrasound showed that the bladder volume was more than 600 mL [8]. The intensity of postoperative pain was evaluated using a visual analog scale (VAS) at rest and on coughing on days 1–4 after the operation, as described previously [9]. Briefly, pain was measured by a 10-point VAS and graded from 0 cm (no pain) to 10 cm (worst pain imaginable). The patients were told to indicate how they felt at rest and on coughing by placing a mark perpendicular to the line. Fasting blood glucose (FBG) was tested in the morning 1 day before the operation (day −1), on day 1, and on day 4 after the operation. Time to first passage of flatus after the surgery (days) and length of hospital stay were recorded. Complications after the operation were recorded. Predetermined discharge criteria were used to measure the length of hospital stay after the operation. The criteria were defined as eating a normal diet, tolerating clear fluids for 24 h, no complaints of pain, no evidence of complications for 24 h, and consent from the patient.
In this clinical trial, postoperative pain at rest on day 1 was determined as the primary outcome. Based on retrospective data from our institution in a similar surgical population, the VAS scores at rest on day 1 after surgery were 2.8 ± 1.4 and 3.9 ± 1.5 in the PCEA group and the PCIA group, respectively. A type I α error of 0.05 and a type II β error of 0.20 indicated that a sample size of 29 patients per group would be necessary to show a difference. To compensate for potential exclusions or withdrawals, we recruited 30 patients in each group.
Statistical analysis was performed using SPSS version 11.5 for Windows (SPSS, Chicago, IL, USA). All data are presented as means ± standard deviations, unless otherwise indicated. Demographic data and the number of patients with complications were analyzed using the χ2 test. VAS scores, FBG, time to first flatus passage, and the length of hospital stay were analyzed using Student’s t-test. A P value of <0.05 was considered to be statistically significant.
Results
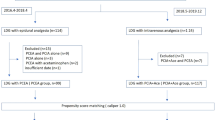
From March 2010 to October 2010, 118 patients undergoing gastric resection for gastric cancer were eligible and 67 patients were included for randomization. Of these 67 patients, 7 did not complete the study because metastasis was discovered at operation and therefore a D2 radical gastric resection for gastric cancer could not be conducted. Thirty patients in each group remained for analysis (Fig. 1). Table 1 shows the clinical features of the 60 patients in the two groups. There were no significant differences in the sex distribution, age, operation and route of reconstruction, or pathological TNM stage between the PCEA group and the PCIA group.
There was a significant difference in the VAS pain scores during analgesia after the operation, as shown in Figs. 2 and 3. Patients treated with PCEA experienced less pain than the PCIA group. The mean VAS pain scores on day 1 and day 2 were 2.9 and 2.3, respectively, in the PCEA group, while the scores in the PCIA group were 3.8 and 3.3, respectively (Fig. 2). In the first 2 days, the VAS scores on coughing were 5.0 and 4.3 in the PCEA group, and 5.9 and 5.4 in the PCIA group (Fig. 3). On day 3 and day 4, after the removal of the PCA pump, the pain scores at rest and on coughing were not significantly different between the two groups, as shown in Figs. 2 and 3.
Visual analog scale (VAS) pain scores at rest on days 1–4 after the operation. On day 1 and day 2 after the operation, there was a significant difference between the PCEA group and the PCIA group (*P < 0.05, †P < 0.01). No significant difference was seen on day 3 or day 4. Boxes represent 25th, 50th, and 75th percentiles, and whiskers the 0–100th percentiles
VAS pain scores on coughing on days 1–4 after the operation. On day 1 and day 2 after the operation, there was a significant difference between the PCEA group and the PCIA group (*P < 0.05). No significant difference was seen on day 3 or day 4. Boxes represent 25th, 50th, and 75th percentiles, and whiskers the 0–100th percentiles
As shown in Fig. 4, all patients in both groups had normal FBG levels before the operation and there were no significant differences between the two groups. On day 1 after the operation, FBG levels in the PCEA group and the PCIA group rose to 4.97 and 5.83 mmol/L, respectively, and in the PCIA group the FBG level was significantly higher than that in the PCEA group (P < 0.05). On day 4 after the operation, the FBG levels in both groups fell and no significant difference could be found between the two groups.
Fasting blood sugar (FBG) on day −1, day 1, and day 4 after the operation. All patients had normal FBG levels before the operation. On day 1 after the operation FBG in the PCEA group was significantly lower than that in the PCIA group (*P < 0.05), while on day 4 after the operation there was no significant difference between the two groups. Boxes represent 25th, 50th, and 75th percentiles, and whiskers the 0–100th percentiles
Time to first passage of flatus after surgery in the PCEA group was 3.1 ± 1.1 days, and in the PCIA group it was 3.9 ± 1.2 days. There was a significant difference between the two groups (P < 0.05, Fig. 5).
There was no surgically related death in either group. Urinary system complications were the most common side effect we saw in the PCEA group. Urinary retention occurred in 4 patients in the PCEA group (13.3 %) and in 1 patient in the PCIA group (3.3 %), and urinary tract infection occurred in 4 patients in the PCEA group (13.3 %) and in 1 patient in the PCIA group (3.3 %). There was no significant difference between the two groups according to the χ2 test (P > 0.05). Pulmonary infection was observed in 1 patient in the PCEA group and 2 patients in the PCIA group. Surgical site infections were observed in 2 patients in the PCEA group and in none of the patients in the PCIA group. Anastomotic leakage was observed in 2 patients in the PCIA group and none of the patients in the PCEA group. Postoperative complications and their incidences are listed in Table 2. There was no significant difference in the incidence of complications between the two groups.
The time to meet the predetermined discharge criteria was 10.7 days in the PCEA group and 11.9 days in the PCIA group. The time in the PCEA group was significantly shorter than that in the PCIA group (P < 0.05, Fig. 6).
Discussion
This randomized clinical trial showed the effect of PCA with epidural bupivacaine–morphine compared with the effect of PCA with intravenous morphine in patients undergoing D2 radical gastrectomy for gastric cancer. The PCEA group, with thoracic epidural anesthesia using bupivacaine and morphine, had superior pain control both at rest and on coughing compared with the PCIA group, who received intravenous morphine. The patients in the PCEA group had better FBG control, which meant these patients experienced less postoperative stress compared with those in the PCIA group. The recovery of gastrointestinal activity was faster and the length of hospital stay was shorter in the PCEA group compared with these parameters in the PCIA group. Of note, we did not see any difference in the incidence of operation-related complications between the two groups.
The application of opioids by epidural analgesia delivers the drug close enough to the spinal cord so that the opioids can inhibit pain transmission from afferent nerves to the central nervous system through interaction with pre- and postsynaptic opioid receptors in the dorsal horn [10, 11]. Many doctors assume that when the same amount of an opioid is used, epidural application of PCA should achieve more effective analgesia than systemic administration. In the present study, though PCA with both epidural bupivacaine–morphine and intravenous morphine provided good analgesia after gastrectomy for gastric cancer, in the first 2 days after the operation we saw lower VAS scores, both at rest and on coughing, in the PCEA group. These results were similar to the results of other prospective randomized controlled studies in different patient populations [4, 6]. Based on previously published literature, epidural analgesia using a local anesthetic combined with an opioid was not only superior in relieving pain at rest and on coughing, but also led to a higher rating of well-being or satisfaction after operation than intravenous opioid analgesia in a wide range of patient populations [5, 12].
Gastrointestinal motility is increased by parasympathetic stimulation. Surgical trauma activates noncholinergic, nonadrenergic spinal reflex mechanisms that block excitatory vagal efferents via bulbar-mediated reflexes [13]. Because of the blocking of this inhibitory reflex when patients use local analgesia with PCEA [14], the combination of an epidural opioid plus bupivacaine may help the recovery of gastrointestinal motility and reduce postoperative ileus [15, 16]. A large retrospective study of 726 Chinese patients after cesarean section showed that the time to first flatus passage in women with epidural PCA was 1.33 days, while the time in women who used intravenous PCA was 1.51 days, which means a quicker recovery of gastrointestinal motility with PCEA [17]. However, that study did not include operations with gastrointestinal excision or anastomosis, and these factors may affect gastrointestinal motility more seriously. The use of opioids has a potential disadvantage because of their adverse effect on gastrointestinal function. Epidural analgesia with bupivacaine, via a low thoracic catheter, inhibits sympathetic outflow from the T5–L2 levels while sparing sacral parasympathetic stimulation. This method of analgesia may increase gastrointestinal motility after an operation and therefore lead to less ileus [18]. Bradshaw et al. [19] demonstrated that standardized perioperative care protocols, including epidural analgesia with bupivacaine or/and morphine, in patients undergoing colon surgery led to a faster return of bowel activity and reduced length of stay in hospital. In our present study, the first time of flatus after gastrectomy for gastric cancer in the patients who used PCEA (with bupivacaine and morphine) was 3.1 days, which was shorter than the time in the patients who used PCIA (with morphine), and there was no significant difference between the two groups in the complications of gastrointestinal obstruction after the operation.
Surgical injury provokes a stress response that leads to a high catabolic state with hyperglycemia and increased oxidation of body protein [20], which is characterized by a state of insulin resistance [21] and can interfere with the postoperative recovery process [22, 23]. In recent years, some perioperative protocols, including preoperative oral carbohydrate [24], epidural analgesia, early nasogastric tube removal, and early feeding with a low-fat liquid diet, have all aimed at suppressing perioperative stress to achieve a faster recovery from surgery and a quicker hospital discharge of patients [19]. Epidural blockade with local anesthetics can facilitate glucose utilization [25] and lessen the loss of body proteins [26]. Yardeni et al. [27], in their randomized controlled trial comparing postoperative pain management techniques, reported an attenuated postoperative increase in serum cortisol and prolactin levels in their PCEA group, which meant that there was diminished activation of the hypothalamic–pituitary–adrenal axis response and a smaller surgery-associated neuroendocrine stress response in the PCEA group. In our study, a lower FBG level was found in patients with PCEA on the first day after gastrectomy for gastric cancer. This meant that there was a smaller stress response in the PCEA group, and this could have contributed to their faster recovery from surgery.
Urine retention is the most common complication with epidural analgesia after surgery. Weiniger et al. [28] reported that 83 % of women with lumbar epidural analgesia for labor required bladder catheterization. Capdevila et al. [29] reported that 53 % of patients with lumbar patient-controlled epidural anesthesia after reconstructive knee surgery had urinary retention in the early postoperative period. In a review of 7357 patients with epidural analgesia in 83 study groups from 1980 to 1999 reported by Dolin et al. [30], 21.5–38.1 % of patients experienced urinary retention. However, the incidence of urinary retention in patients with PCEA seemed to be lower in some recent studies. In a study by Ferguson et al. [4], a prospective randomized trial comparing PCEA with intravenous analgesia after major open gynecologic cancer surgery, only about 10 % of patients experienced urinary retention. Their results were similar to ours. Ladak et al. [8] found that, in patients with thoracic patient-controlled epidural analgesia (TPCEA) undergoing thoracotomy, the incidence of postoperative urine retention was about 10 %, which was also similar to the results of our study. Their study also showed that the incidence of urine retention did not have an association with catheter level (T3–T6 vs. T6–T8), drug type (bupivacaine 0.1 % + hydromorphone 0.015 mg/mL vs. ropivacaine 0.2 %), or infusion rate, nor did the incidence of urine retention have an association with the age, gender, or weight of the patients. An interesting trial was done by Chia et al. [23], comparing the incidence of urine retention after thoracotomy under postoperative PCEA in patients for whom the transurethral catheter was removed on the first postoperative day and those for whom the catheter was removed after the discontinuation of PCEA. They found that after removal of the bladder catheter, no patient in either group required re-catheterization for urinary retention and no patient had a catheter-related infection and they concluded that routine continuous bladder catheterization might not necessarily be required after thoracotomy in patients undergoing continuous thoracic epidural analgesia. In our study, we removed the catheter 24 h after the end of the PCA, and we did not find any difference in the incidence of urine retention between the PCEA and the PCIA groups.
In our study, the length of stay in hospital after gastrectomy in the PCEA group was 10.7 ± 1.7 days, which was significantly shorter than that in the PCIA group (11.9 ± 1.8 days). Less postoperative pain, a lower stress response, and a quicker return of bowel activity may all have contributed to the faster recovery from surgery in the PCEA group compared with the PCIA group.
In conclusion, our findings reveal that PCEA is safe and effective in patients undergoing gastrectomy for gastric cancer. PCEA results in a lower pain VAS score, a lower stress response, a quicker return of bowel activity, and a faster recovery than PCIA after gastrectomy for gastric cancer.
References
Xue YW, Wei YZ. The relationship of prognosis to surgery and pathologic characteristics of stage IV (M0) gastric cancer patients. Chin J Cancer. 2010;29:355–8.
Weissman C. The metabolic response to stress: an overview and update. Anesthesiology. 1990;73:308–27.
Ahn H, Bronge A, Johansson K, Ygge H, Lindhagen J. Effect of continuous postoperative epidural analgesia on intestinal motility. Br J Surg. 1988;75:1176–8.
Ferguson SE, Malhotra T, Seshan VE, Levine DA, Sonoda Y, Chi DS, et al. A prospective randomized trial comparing patient-controlled epidural analgesia to patient-controlled intravenous analgesia on postoperative pain control and recovery after major open gynecologic cancer surgery. Gynecol Oncol. 2009;114:111–6.
Weber T, Matzl J, Rokitansky A, Klimscha W, Neumann K, Deusch E. Superior postoperative pain relief with thoracic epidural analgesia versus intravenous patient-controlled analgesia after minimally invasive pectus excavatum repair. J Thorac Cardiovasc Surg. 2007;134:865–70.
Miro M, Guasch E, Gilsanz F. Comparison of epidural analgesia with combined spinal-epidural analgesia for labor: a retrospective study of 6497 cases. Int J Obstet Anesth. 2008;17:15–9.
Ohtsu A. Diverse eastern and western approaches to the management of gastric cancer. Gastrointest Cancer Res. 2007;1:S10–5.
Ladak SS, Katznelson R, Muscat M, Sawhney M, Beattie WS, O’Leary G. Incidence of urinary retention in patients with thoracic patient-controlled epidural analgesia (TPCEA) undergoing thoracotomy. Pain Manag Nurs. 2009;10:94–8.
Niiyama Y, Kawamata T, Shimizu H, Omote K, Namiki A. The addition of epidural morphine to ropivacaine improves epidural analgesia after lower abdominal surgery. Can J Anaesth. 2005;52:181–5.
Lombard MC, Besson JM. Attempts to gauge the relative importance of pre- and postsynaptic effects of morphine on the transmission of noxious messages in the dorsal horn of the rat spinal cord. Pain. 1989;37:335–45.
Sivilotti LG, Gerber G, Rawat B, Woolf CJ. Morphine selectively depresses the slowest, NMDA-independent component of C-fibre-evoked synaptic activity in the rat spinal cord in vitro. Eur J Neurosci. 1995;7:12–8.
Saeki H, Ishimura H, Higashi H, Kitagawa D, Tanaka J, Maruyama R, et al. Postoperative management using intensive patient-controlled epidural analgesia and early rehabilitation after an esophagectomy. Surg Today. 2009;39:476–80.
Glise H, Abrahamsson H. Reflex inhibition of gastric motility pathophysiological aspects. Scand J Gastroenterol Suppl. 1984;89:77–82.
de Leon-Casasola OA, Karabella D, Lema MJ. Bowel function recovery after radical hysterectomies: thoracic epidural bupivacaine–morphine versus intravenous patient-controlled analgesia with morphine: a pilot study. J Clin Anesth. 1996;8:87–92.
Liu SS, Carpenter RL, Mackey DC, Thirlby RC, Rupp SM, Shine TS, Feinglass NG, Metzger PP, Fulmer JT, Smith SL. Effects of perioperative analgesic technique on rate of recovery after colon surgery. Anesthesiology. 1995;83:757–65.
Bredtmann RD, Herden HN, Teichmann W, Moecke HP, Kniesel B, Baetgen R, et al. Epidural analgesia in colonic surgery: results of a randomized prospective study. Br J Surg. 1990;77:638–42.
Liu YF, Chen KB, Lin HL, Ho CH, Liu SK, Liu YC, et al. Comparison of the effect of epidural and intravenous patient-controlled analgesia on bowel activity after cesarean section: a retrospective study of 726 Chinese patients. Acta Anaesthesiol Taiwan. 2009;47:22–7.
Steinbrook RA. Epidural anesthesia and gastrointestinal motility. Anesth Analg. 1998;86:837–44.
Bradshaw BG, Liu SS, Thirlby RC. Standardized perioperative care protocols and reduced length of stay after colon surgery. J Am Coll Surg. 1998;186:501–6.
Wilmore DW. Catabolic illness. Strategies for enhancing recovery. N Engl J Med. 1991;325:695–702.
Thorell A, Efendic S, Gutniak M, Haggmark T, Ljungqvist O. Insulin resistance after abdominal surgery. Br J Surg. 1994;81:59–63.
Watters JM, Clancey SM, Moulton SB, Briere KM, Zhu JM. Impaired recovery of strength in older patients after major abdominal surgery. Ann Surg. 1993;218:380–390; discussion 390–383.
Chia YY, Wei RJ, Chang HC, Liu K. Optimal duration of urinary catheterization after thoracotomy in patients under postoperative patient-controlled epidural analgesia. Acta Anaesthesiol Taiwan. 2009;47:173–9.
Klotz S, Danser AH, Foronjy RF, Oz MC, Wang J, Mancini D, et al. The impact of angiotensin-converting enzyme inhibitor therapy on the extracellular collagen matrix during left ventricular assist device support in patients with end-stage heart failure. J Am Coll Cardiol. 2007;49:1166–74.
Schricker T, Wykes L, Carli F. Epidural blockade improves substrate utilization after surgery. Am J Physiol Endocrinol Metab. 2000;279:E646–53.
Vedrinne C, Vedrinne JM, Guiraud M, Patricot MC, Bouletreau P. Nitrogen-sparing effect of epidural administration of local anesthetics in colon surgery. Anesth Analg. 1989;69:354–9.
Yardeni IZ, Shavit Y, Bessler H, Mayburd E, Grinevich G, Beilin B. Comparison of postoperative pain management techniques on endocrine response to surgery: a randomised controlled trial. Int J Surg. 2007;5:239–43.
Weiniger CF, Wand S, Nadjari M, Elchalal U, Mankuta D, Ginosar Y, et al. Post-void residual volume in labor: a prospective study comparing parturients with and without epidural analgesia. Acta Anaesthesiol Scand. 2006;50:1297–303.
Capdevila X, Barthelet Y, Biboulet P, Ryckwaert Y, Rubenovitch J, d’Athis F. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology. 1999;91:8–15.
Dolin SJ, Cashman JN. Tolerability of acute postoperative pain management: nausea, vomiting, sedation, pruritus, and urinary retention. Evidence from published data. Br J Anaesth. 2005;95:584–91.
Author information
Authors and Affiliations
Corresponding author
Additional information
Z. Zhu, C. Wang, and C. Xu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhu, Z., Wang, C., Xu, C. et al. Influence of patient-controlled epidural analgesia versus patient-controlled intravenous analgesia on postoperative pain control and recovery after gastrectomy for gastric cancer: a prospective randomized trial. Gastric Cancer 16, 193–200 (2013). https://doi.org/10.1007/s10120-012-0168-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-012-0168-z