Abstract
Background
In cancer patients, impaired function of immune cells—such as CD8+ T cells, NK cells, and dendritic cells—reportedly results in tumor progression. Although γδ T cells also play a critical role in tumor defense, their function remains unclear in cancer patients.
Methods
The frequency and function of γδ T cells in peripheral blood, normal gastric mucosa, and cancer tissue were evaluated by multicolor flow cytometry. We also determined NKG2D expression on γδ T cells in gastric cancer patients.
Results
The frequency of Vδ1 γδ T cells in gastric cancer tissue is significantly lower than in normal gastric mucosa; however, differences in the frequencies of Vδ2 and Vγ9 γδ T cells between normal gastric mucosa and gastric cancer tissue were not statistically significant. The Vδ1 γδ T cells from gastric cancer tissue produce significantly less IFN-γ than those from normal gastric mucosa do. Expression of NKG2D on Vδ1 γδ T cells from gastric cancer tissue was significantly lower than in normal gastric mucosa. We also found a significant correlation between NKG2D expression and IFN-γ production of Vδ1 γδ T cells in gastric cancer tissue.
Conclusion
Vδ1 γδ T cells show decreased frequency and impaired function in gastric cancer tissue, for which decreased NKG2D expression might be one of the mechanisms. Modalities specifically targeting NKG2D in Vδ1 γδ T cells may provide a breakthrough treatment for gastric cancer patients.
Similar content being viewed by others
Introduction
T lymphocytes recognize antigens via the heterodimeric T-cell receptor (TCR) molecule, which is noncovalently associated with the CD3 molecular complex. T lymphocytes are classified into two subsets, based on their TCR. Most T cells carry TCRs thay consist of an αβ chain heterodimer. These T cells, referred to as αβ T cells, can be further subdivided on the basis of the surface markers CD4 and CD8 [1].
γδ T cells are another type of T cell; these account for 2–5% of CD3+ peripheral blood T cells but constitute a dominant fraction of the T cells at other anatomical sites, such as the intestinal epithelia. Most γδ T cells lack CD4 or CD8 antigens and hence display a “double-negative” phenotype, although a sizeable fraction express CD8. The absence of CD4 or CD8 expression on the majority of circulating γδ T cells is well in line with the lack of MHC restriction in the antigen recognition of this T-cell subset [2]. In the blood of most healthy individuals, T cells expressing the Vδ2 gene paired with a particular Vγ chain (Vγ9) account for 50 to >90% of the γδ T-cell population. In contrast, intestinal intraepithelial γδ T cells frequently express the Vδ1 gene, which can associate with different Vγ elements [2]. There are no major differences between αβ and γδ T cells with regard to effector functions. Thus, activated γδ T cells have strong cytotoxic effector activity (using both death receptor/death ligand and cytolytic granule pathways), and produce various cytokines (frequently including tumor necrosis factor-α and IFN-γ) [2]. However, γδ TCRs seem to react to novel ligands that are not recognized by αβ T cells, thus providing an essential additional pathway of local immunosurveillance with immediate relevance for tumor defense [2, 3].
There is accumulating evidence that immune cells such as CD8+ T cells, NK cells, and dendritic cells show impaired function in cancer patients, resulting in tumor progression [4]. However, the function of γδ T cells in cancer patients is unclear. The critical role of γδ T cells in tumor defense implies that γδ T-cell function is also impaired by cancer cells. In the current study, we therefore examined the frequency and function of γδ T cells in peripheral blood, normal gastric mucosa, and cancer tissue. We also determined NKG2D expression on γδ T cells in gastric cancer patients to show a mechanism that impairs γδ T-cell function, because NKG2D is required by γδ T cells to recognize and kill tumor cells.
Materials and methods
Patients and normal donors
Forty-seven patients (37 males and 10 females), treated at Tottori University Hospital and diagnosed with gastric cancer by pathologists, were enrolled in this study. None of the patients received radiotherapy, chemotherapy, or other medical interventions before blood donations. Informed consent for blood donations was obtained from all participants. Patient characteristics are shown in Table 1. Healthy controls (n = 23; 16 males and 7 females) were age-matched (67.9 ± 11.0 years for controls; 69.6 ± 10.8 years for cancer patients), and each experiment was performed in parallel. The clinicopathological findings were determined according to the Japanese Classification of Gastric Carcinoma [5].
Preparation of peripheral blood mononuclear cells (PBMCs)
Approximately 30 ml of peripheral blood was drawn from each control, and from each patient before surgery or chemotherapy; blood samples were prepared by centrifugation over a Ficoll-Paque (Pharmacia, Uppsala, Sweden) density gradient.
Isolation of tumor-infiltrating leukocytes
Freshly excised tumor tissues were minced and incubated in 1.5 mg/ml of collagenase D (Wako Pure Chemical Industries, Ltd., Osaka, Japan). Cell suspensions were then filtered through a mesh filter (BD Falcon, Franklin Lakes, NJ, USA). Tumor-infiltrating leukocytes (TILs) were available in 26 patients for the subset analysis of γδ T cells and in 12 patients for the analysis of NKG2D expression and IFN-γ production of Vδ1 γδ T cells. Since it is necessary to get large piece of tissue sample to have enough TILs for flow cytometry analysis, TILs from most early gastric cancer patients were not available for the analysis of NKG2D expression and IFN-γ production of Vδ1 γδ T cells.
Flow cytometry analysis
Flow cytometry analysis was performed on a FACSCalibur™ (Becton Dickinson, Franklin Lakes, NJ, USA), using the following antibodies: anti-CD3-PE, anti-CD8-PE-Cy5, anti-CD3-PE-Cy5, anti-NKG2D-PE, anti-TCR γδ-FITC (BD PharMingen, Franklin Lakes, NJ, USA), anti-Vδ1-FITC, anti-Vδ2-FITC (Thermo Scientific, Waltham, MA, USA), anti-Vγ9-FITC monoclonal antibody(mAb) (Beckman Coulter, Brea, CA, USA). For intracellular cytokine staining, PBMCs were cultured in the presence of Leukocyte Activation Cocktail (BD PharMingen). The anti-cytokine antibody was anti-IFN-γ-PE (Becton Dickinson). To stain for IFN-γ, cells were fixed and permeabilized with BD Cytofix/Cytoperm™ solution (Becton Dickinson).
Statistical analysis
Either paired t tests or Mann–Whitney U tests were used to determine statistical differences between groups. Correlations were analyzed using the Spearman rank correlation coefficient. P < 0.05 was considered significant. GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA) was used for statistical analyses.
Results
Prevalence of circulating γδ T cells in gastric cancer patients
We first determined the frequency of each subtype of γδ T cells in the peripheral blood of healthy controls and gastric cancer patients. Frequencies of Vδ1, Vδ2, and Vγ9 γδ T cells in healthy controls were 1.9 ± 1.3, 3.8 ± 3.4, and 4.1 ± 3.5%, respectively. Those in gastric cancer patients were 1.8 ± 1.5, 2.6 ± 3.5, and 2.7 ± 3.6%, respectively. The frequency of Vγ9 γδ T cells in gastric cancer patients was significantly lower than in healthy controls (P = 0.048). Furthermore, the frequency of Vδ2 in gastric cancer patients tended to be lower than that in healthy controls (Fig. 1).
Frequencies of Vδ1, Vδ2, and Vγ9 γδ T cells in peripheral blood from healthy control and gastric cancer patients. The frequency of Vγ9 γδ T cells in gastric cancer patients was significantly lower than that in healthy controls (P = 0.048); the frequency of Vδ2 cells in gastric cancer patients also tended to be lower than that in healthy controls (P = 0.095)
Frequency of γδ T cells in the tissue of normal gastric mucosa and gastric cancer
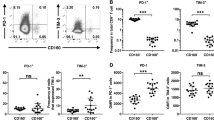
We then investigated the frequencies of Vδ1, Vδ2, and Vγ9 γδ T cells in both normal gastric mucosa and gastric cancer tissue. The frequencies of Vδ1, Vδ2, and Vγ9 γδ T cells in normal gastric mucosa were 51.4 ± 15.3, 4.9 ± 4.6, and 2.7 ± 3.0%, respectively (Fig. 2a). The frequencies of Vδ1, Vδ2, and Vγ9 γδ T cells in gastric cancer tissue were 41.4 ± 16.0, 3.2 ± 2.9, and 2.4 ± 2.5%, respectively (Fig. 2b). The frequency of Vδ1 γδ T cells in gastric cancer tissue was significant lower than that in normal gastric mucosa (P = 0.015; Fig. 2c). However, there were no statistically significant differences in the frequencies of Vδ2 and Vγ 9 γδ T cells between normal gastric mucosa and gastric cancer tissue. On the other hand, the frequency of Vδ1 γδ T cells in gastric cancer tissue was 41.0 ± 8.8 and 41.6 ± 18.5% in early and advanced gastric cancer, respectively, and this difference was not significant (P = 0.98). Furthermore, the frequency of Vδ1 γδ T cells in gastric cancer tissue was 37.2 ± 11.1 and 45.0 ± 18.9% in stages I/II and III/IV, respectively, and this difference was not significant (P = 0.23).
Frequencies of Vδ1, Vδ2, and Vγ9 γδ T cells in both normal gastric mucosa (a) and gastric cancer tissue (b). c The frequency of Vδ1 γδ T cells in gastric cancer tissue was significantly less than that in normal gastric mucosa (P = 0.015). On the other hand, there were no statistically significant differences in the frequencies of Vδ2 and Vγ9 γδ T cells between normal gastric mucosa and gastric cancer tissue
NKG2D expression on Vδ1 γδ T cells decreased in gastric cancer tissue
Our data showed that γδ T cells are abundant in gastric cancer tissue compared with peripheral blood. Moreover, because Vδ1 γδ T cells predominate among γδ T cells in gastric cancer tissue, we investigated the function of such cells in gastric cancer patients. There was no statistical difference in IFN-γ production from circulating Vδ1 γδ T cells between normal controls and cancer patients. On the other hand, Vδ1 γδ T cells obtained from gastric cancer tissue produced significantly less IFN-γ than those obtained from normal gastric mucosa (P = 0.0097; Fig. 3). Since γδ T cells require NKG2D to recognize and kill target cells, we examined NKG2D expression on Vδ1 γδ T cells in both normal gastric mucosa and gastric cancer tissue, and found it to be 60.0 ± 13.8 and 45.6 ± 10.1%, respectively; this difference was statistically significant (P = 0.0009; Fig. 4). With regard to the correlation between NKG2D expression and IFN-γ production of Vδ1 γδ T cells, IFN-γ was mainly produced by NKG2D-expressing Vδ1 γδ T cells (Fig. 5a). Furthermore, we also found that the NKG2D expression and IFN-γ production of Vδ1 γδ T cells in gastric cancer tissue were significantly correlated (r = 0.75, P = 0.007; Fig. 5b).
Comparison of IFN-γ production by Vδ1 γδ T cells from normal gastric mucosa and from gastric cancer tissue. a The representative results for IFN-γ production by Vδ1 γδ T cells from normal gastric mucosa and from gastric cancer tissue by FACS. b Vδ1 γδ T cells from gastric cancer tissue produced significantly less IFN-γ than those from normal gastric mucosa (P = 0.0097)
Comparison of NKG2D expression on Vδ1 γδ T cells in normal gastric mucosa and gastric cancer tissue. a The representative results for NKG2D expression on Vδ1 γδ T cells from normal gastric mucosa and from gastric cancer tissue by FACS. b NKG2D expression on Vδ1 γδ T cells in gastric cancer tissue was significantly lower than in normal gastric mucosa (P = 0.0009)
Correlation between NKG2D expression and IFN-γ production of Vδ1 γδ T cells in gastric cancer tissue. a The representative results for the correlation between NKG2D expression and IFN-γ production of Vδ1 γδ T cells from gastric cancer tissue by FACS. Cells are gated on CD3+ Vδ1+ cells. b IFN-γ production of Vδ1 γδ T cells was significantly related to NKG2D expression on Vδ1 γδ T cells (r = 0.75, P = 0.007)
Discussion
In the current study, we demonstrated that Vδ1 γδ T cells predominate among γδ T cells in both normal gastric mucosa and gastric cancer, whereas Vδ2 and Vγ9 γδ T cells predominate in peripheral blood. The Vδ1 γδ T cells isolated from TILs of patients with colorectal cancer have been shown to lyse not only autologous but also various allogeneic epithelial tumor cells, indicating the importance of Vδ1 γδ T cells in tumor surveillance [6]. In the current study, we found that the frequency of Vδ1 γδ T cells in gastric cancer tissue was significantly less than in normal gastric mucosa. Furthermore, IFN-γ production by Vδ1 γδ T cells in gastric cancer tissue was significantly less than that in normal gastric mucosa, indicating that Vδ1 γδ T cell function in gastric cancer tissue is impaired. On the other hand, the frequency of Vδ1 γδ T cells in the tissue of gastric cancer was not related to disease progression. Since we determined the frequency of Vδ1 γδ T cells in the tissue of gastric cancer, those cells are considered to have been already affected by cancer cells and their stromal cells, even in early gastric cancer.
The MHC class I chain-related molecules A and B (MICA and MICB) and the UL-16 binding proteins 1–3 (ULBP1–3) can be induced on epithelial cells by heat shock or oxidative stress, and are constitutively expressed to variable levels on many epithelial tumor cells and also on some leukemias and lymphomas. Such MICA/MICB- or ULBP-expressing tumor and lymphoma cells are killed by Vδ1 γδ T cells through recognition by NKG2D, which is the receptor for MICA, MICB, and ULBP [7, 8]. NKG2D is a type II C-lectin-like protein encoded by a gene located next to the NKG2A, NKG2C, and NKG2E genes within the natural killer (NK) gene complex on human chromosome 12p12–p13 and mouse chromosome 6 [9]. NKG2D is an activating cell surface receptor expressed by NK cells, γδ T cells, some cytolytic CD8+ αβ T cells, and NKT cells [10–13]. In cancer patients, tumor-infiltrating and systemic NK cells and CD8+ T cells often express little NKG2D and are functionally compromised, indicating that decreased NKG2D expression on NK cells and CD8+ T cells is closely related to impaired function of those cells [14]. However, NKG2D expression on γδ T cells and its relationship to impaired γδ T-cell function in cancer patients remains unclear. In the current study, therefore, we studied NKG2D expression in Vδ1 γδ T cells to identify a mechanism responsible for impaired γδ T-cell function in gastric cancer patients. NKG2D expression on Vδ1 γδ T cells is significantly lower in gastric cancer tissue than in normal gastric mucosa. Furthermore, in gastric cancer tissue, NKG2D expression in Vδ1 γδ T cells significantly correlates with IFN-γ production by Vδ1 γδ T cells, indicating that the impaired γδ T-cell function observed in these tissues is due to decreased NKG2D expression on Vδ1 γδ T cells. The reason for this phenomenon remains unclear. We previously showed that NKG2D expression on CD8+ T cells is also downregulated; this decreased NKG2D expression correlates significantly with reduced IFN-γ production by CD8+ T cells [15]. We have also shown that MICA expressed in gastric cancer cells is responsible for decreased NKG2D expression on CD8+ T cells. Further studies are urgently required to solve this problem.
Immune cells with negative immune regulatory function at tumor sites have recently attracted considerable attention, since these cells are thought to play extremely important roles in the progression of tumors through the suppression of immune responses and induction of immune tolerance. The best-studied immunosuppressive cell population is that of CD4+/CD25+ regulatory T cells (Treg cells) [16–18]. Human Treg cells [19, 20] disable TAA-specific T-cell immunity [21, 22]. Increased numbers of Treg cells are correlated with poor survival in hepatocellular carcinoma patients [23] and ovarian cancer patients [19]. Treg cells also increase in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric cancer [24]. Furthermore, myeloid-derived suppressor cells [25, 26] and CD4+/NKG2D+ T cells [27] can diminish immunity against cancer cells in cancer patients. These accumulating lines of evidence indicate that immunosuppressive cells play important roles in tumor progression. Notably, γδ T cells also have an immune regulatory function. In this regard, Peng et al. [28] showed that a dominant Vδ1 γδ T cell population among lymphocytes infiltrating breast tumors was strongly able to suppress naive and effector T-cell responses, and to block the maturation and function of dendritic cells. Furthermore, they showed that these cells have suppressive activity in vivo through adoptive cotransfer experiments. Although the dominant subpopulation of γδ T cells in gastric cancer tissue was also Vδ1 γδ T cells in the current study, the frequency of Vδ1 γδ T cells in the tissue of gastric cancer was significantly lower than that in normal gastric mucosa. Therefore, it is unlikely that the abundant Vδ1 γδ T cells seen in the current study have an immune regulatory function, although their immune regulatory activity was not determined in the current study.
In conclusion, our results show that the frequency of Vδ1 γδ T cells was decreased and that their function was impaired in gastric cancer tissue. Decreased NKG2D expression may be one of the mechanisms responsible for impaired Vδ1 γδ T-cell function. Development of specific treatments targeting NKG2D on Vδ1 γδ T cells may provide a clinical breakthrough for gastric cancer patients.
References
Kabelitz D, Wesch D, Pitters E, Zoller M. Potential of human gammadelta T lymphocytes for immunotherapy of cancer. Int J Cancer. 2004;112:727–32.
Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026.
Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, et al. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–9.
Whiteside TL. Immune suppression in cancer: effects on immune cells, mechanisms and future therapeutic intervention. Semin Cancer Biol. 2006;16:3–15.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma—2nd English edition. Gastric Cancer. 1998;1:10–24.
Maeurer MJ, Martin D, Walter W, Liu K, Zitvogel L, Halusczcak K, et al. Human intestinal Vdelta1+ lymphocytes recognize tumor cells of epithelial origin. J Exp Med. 1996;183:1681–96.
Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci USA. 1999;96:6879–84.
Poggi A, Venturino C, Catellani S, Clavio M, Miglino M, Gobbi M, et al. Vdelta1 T lymphocytes from B-CLL patients recognize ULBP3 expressed on leukemic B cells and up-regulated by trans-retinoic acid. Cancer Res. 2004;64:9172–9.
Glienke J, Sobanov Y, Brostjan C, Steffens C, Nguyen C, Lehrach H, et al. The genomic organization of NKG2C, E, F, and D receptor genes in the human natural killer gene complex. Immunogenetics. 1998;48:163–73.
Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–90.
Watzl C. The NKG2D receptor and its ligands-recognition beyond the “missing self”? Microbes Infect. 2003;5:31–7.
Vivier E, Tomasello E, Paul P. Lymphocyte activation via NKG2D: towards a new paradigm in immune recognition? Curr Opin Immunol. 2002;14:306–11.
Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–9.
Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–8.
Osaki T, Saito H, Yoshikawa T, Matsumoto S, Tatebe S, Tsujitani S, et al. Decreased NKG2D expression on CD8+ T cell is involved in immune evasion in patients with gastric cancer. Clin Cancer Res. 2007;13:382–7.
Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400.
Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32.
von Herrath MG, Harrison LC. Antigen-induced regulatory T cells in autoimmunity. Nat Rev Immunol. 2003;3:223–32.
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9.
Woo EY, Yeh H, Chu CS, Schlienger K, Carroll RG, Riley JL, et al. Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168:4272–6.
Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–74.
Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307.
Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–39.
Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res. 2003;9:4404–8.
Ochoa AC, Zea AH, Hernandez C, Rodriguez PC. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res. 2007;13:721s–6s.
Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–43.
Groh V, Smythe K, Dai Z, Spies T. Fas-ligand-mediated paracrine T cell regulation by the receptor NKG2D in tumor immunity. Nat Immunol. 2006;7:755–62.
Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, Wang RF. Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity. 2007;27:334–48.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuroda, H., Saito, H. & Ikeguchi, M. Decreased number and reduced NKG2D expression of Vδ1 γδ T cells are involved in the impaired function of Vδ1 γδ T cells in the tissue of gastric cancer. Gastric Cancer 15, 433–439 (2012). https://doi.org/10.1007/s10120-011-0138-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-011-0138-x