Abstract
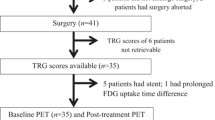
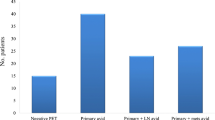
Perioperative chemotherapy in stage II and stage III gastric cancer is now accepted as a standard of care in the Western world. Two randomized phase III studies have shown improved survival for patients with induction chemotherapy followed by surgery compared with surgery alone. It is generally accepted that patients who respond to induction therapy have a significantly improved survival compared with that in nonresponding patients. Unfortunately no prospectively tested markers predicting response and/or prognosis are available for clinical practice. In adenocarcinomas of the esophagogastric junction (AEG), fluorodeoxyglucose-positron emission tomography (FDG-PET) prospectively was established as a surrogate predicting response and prognosis. The MUNICON (Metabolic response evalUatioN for Individualisation of neoadjuvant Chemotherapy in oesOphageal and oesophagogastric adeNocarcinoma) I study confirmed prospectively the usefulness of early metabolic response evaluation and showed the feasibility of a PET-guided treatment algorithm. These findings are an important step forward in the tailoring of multimodal treatment in accordance with tumor biology. In gastric cancer, we have analyzed FDG-PET in a prospective study. In gastric cancer the issue is more complicated, because about 30% of gastric cancers cannot be visualized with sufficient contrast for quantification. Insufficient FDG uptake is mostly associated with diffuse-type gastric cancer with signet ring cells and mucinous content. In FDG-avid patients, FDG-PET can be used for response evaluation, comparable to that in AEG. The prognosis of FDG-nonavid patients is similar to that in metabolic nonresponders. The addition of new tracers such as fluorothymidine may increase the sensitivity of PET in the future. Treatment concepts such as immediate resection after only 2 weeks of induction therapy with or without adjuvant treatment could be considered in metabolic nonresponders, or modified chemotherapy regimens, possibly including biologically targeted drugs, could be considered in those with FDG-nonavid tumors.
Article PDF
Similar content being viewed by others
References
Roder JD, Bottcher K, Siewert JR, Busch R, Hermanek P, Meyer HJ. Prognostic factors in gastric carcinoma. Results of the German Gastric Carcinoma Study 1992. Cancer 1993;72:2089–2097.
Bonenkamp JJ, Hermans J, Sasako M, Van de Velde CJ, Welvaart K, Songun I, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med 1999;340:908–914.
Hartgrink HH, Van de Velde CJ, Putter H, Bonenkamp JJ, Klein KE, Songun I, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch Gastric Cancer Group trial. J Clin Oncol 2004;22:2069–2077.
Hartgrink HH, Bonenkamp HJ, Van d, V. Influence of surgery on outcomes in gastric cancer. Surg Oncol Clin N Am 2000;9:97–98.
Ott K, Sendler A, Becker K, Dittler HJ, Helmberger H, Busch R, et al. Neoadjuvant chemotherapy with cisplatin, 5-FU, and leucovorin (PLF) in locally advanced gastric cancer: a prospective phase II study. Gastric Cancer 2003;6:159–167.
Schuhmacher CP, Fink U, Becker K, Busch R, Dittler HJ, Mueller J, et al. Neoadjuvant therapy for patients with locally advanced gastric carcinoma with etoposide, doxorubicin, and cisplatinum. Closing results after 5 years of follow-up. Cancer 2001;91:918–927.
Ajani JA, Mansfield PF, Lynch PM, Pisters PW, Feig B, Dumas P, et al. Enhanced staging and all chemotherapy preoperatively in patients with potentially resectable gastric carcinoma. J Clin Oncol 1999;17:2403–2411.
Ajani JA, Komaki R, Putnam JB, Walsh G, Nesbitt J, Pisters PW, et al. A three-step strategy of induction chemotherapy then chemoradiation followed by surgery in patients with potentially resectable carcinoma of the esophagus or gastroesophageal junction. Cancer 2001;92:279–286.
Ott K, Vogelsang H, Mueller J, Becker K, Muller M, Fink U, et al. Chromosomal instability rather than p53 mutation is associated with response to neoadjuvant cisplatin-based chemotherapy in gastric carcinoma. Clin Cancer Res 2003;9:2307–2315.
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11–20.
Boige V, Pignon J, Saint-Aubert B, Lasser P, Conroy T, Bouche O, et al. Final results of a randomized trial comparing preoperative 5-fluorouracil (F)/cisplatin (P) to surgery alone in adenocarcinoma of stomach and lower esophagus (ASLE): FNLCC ACCORD07-FFCD 9703 trial (Meeting Abstract). J Clin Oncol 2007;25:4510.
Lowy AM, Mansfield PF, Leach SD, Pazdur R, Dumas P, Ajani JA. Response to neoadjuvant chemotherapy best predicts survival after curative resection of gastric cancer. Ann Surg 1999;229:303–308.
Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981;47:207–214.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–216.
Park JO, Lee SI, Song SY, Kim K, Kim WS, Jung CW, et al. Measuring response in solid tumors: comparison of RECIST and WHO response criteria. Jpn J Clin Oncol 2003;33:533–537.
Sasaki T. New guidelines to evaluate the response to treatment “RECIST” (in Japanese). Gan To Kagaku Ryoho 2000;27:2179–2184
Yoshida S, Miyata Y, Ohtsu A, Boku N, Shirao K, Shimada Y. Significance of and problems in adopting response evaluation criteria in solid tumor RECIST for assessing anticancer effects of advanced gastric cancer. Gastric Cancer 2000;3:128–133.
Jeung HC, Rha SY, Noh SH, Roh JK, Chung HC. A phase II trial of weekly fractionated irinotecan and cisplatin for advanced gastric cancer. Cancer Chemother Pharmacol 2007;59:313–320.
Burge ME, Smith D, Topham C, Jackson DP, Anthoney DA, Halstead F, et al. A phase I and II study of 2-weekly irinotecan with capecitabine in advanced gastroesophageal adenocarcinoma. Br J Cancer 2006;94:1281–1286.
Ott K, Fink U, Becker K, Stahl A, Dittler HJ, Busch R, et al. Prediction of response to preoperative chemotherapy in gastric carcinoma by metabolic imaging: results of a prospective trial. J Clin Oncol 2003;21:4604–4610.
Weber WA, Ott K, Becker K, Dittler HJ, Helmberger H, Avril NE, et al. Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol 2001;19:3058–3065.
Ott K, Weber WA, Lordick F, Becker K, Busch R, Herrmann K, et al. Metabolic imaging predicts response, survival, and recurrence in adenocarcinomas of the esophagogastric junction. J Clin Oncol 2006;24:4692–4698.
Swisher SG, Maish M, Erasmus JJ, Correa AM, Ajani JA, Bresalier R, et al. Utility of PET, CT, and EUS to identify pathologic responders in esophageal cancer. Ann Thorac Surg 2004;78:1152–1160.
Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994;73:2680–2686.
Becker K, Mueller JD, Schuhmacher C, Ott K, Fink U, Busch R, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 2003;98:1521–1530.
Rohatgi P, Swisher SG, Correa AM, Wu TT, Liao Z, Komaki R, et al. Characterization of pathologic complete response after preoperative chemoradiotherapy in carcinoma of the esophagus and outcome after pathologic complete response. Cancer 2005;104:2365–2372.
Swisher SG, Hofstetter W, Wu TT, Correa AM, Ajani JA, Komaki RR, et al. Proposed revision of the esophageal cancer staging system to accommodate pathologic response (pP) following preoperative chemoradiation (CRT). Ann Surg 2005;241:810–817.
Shah MA, Yeung HW, Coit D, Trocola R, Ilson D, Randazzo J, et al. A phase II study of preoperative chemotherapy with irinotecan (CPT) and cisplatin (CIS) for gastric cancer (NCI 5917): FDG-PET/CT predicts patient outcome (Meeting Abstract). J Clin Oncol 2007;25:4502.
Lordick F, Ott K, Krause BJ, Weber WA, Becker K, Stein HJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol 2007;8:797–805.
Siewert JR, Lordick F, Ott K, Stein HJ, Weber WA, Becker K, et al. Induction chemotherapy in Barrett cancer: influence on surgical risk and outcome. Ann Surg 2007;246:624–631.
Beer AJ, Wieder HA, Lordick F, Ott K, Fischer M, Becker K, et al. Adenocarcinomas of esophagogastric junction: multidetector row CT to evaluate early response to neoadjuvant chemotherapy. Radiology 2006;239:472–480.
Wieder HA, Beer AJ, Lordick F, Ott K, Fischer M, Rummeny EJ, et al. Comparison of changes in tumor metabolic activity and tumor size during chemotherapy of adenocarcinomas of the esophagogastric junction. J Nucl Med 2005;46:2029–2034.
Stahl A, Ott K, Weber WA, Becker K, Link T, Siewert JR, et al. FDG PET imaging of locally advanced gastric carcinomas: correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging 2003;30:288–295.
Mukai K, Ishida Y, Okajima K, Isozaki H, Morimoto T, Nishiyama S. Usefulness of preoperative FDG-PET for detection of gastric cancer. Gastric Cancer 2006;9:192–196.
Kim SK, Kang KW, Lee JS, Kim HK, Chang HJ, Choi JY, et al. Assessment of lymph node metastases using 18F-FDG PET in patients with advanced gastric cancer. Eur J Nucl Med Mol Imaging 2006;33:148–155.
Chen J, Cheong JH, Yun MJ, Kim J, Lim JS, Hyung WJ, et al. Improvement in preoperative staging of gastric adenocarcinoma with positron emission tomography. Cancer 2005;103:2383–2390.
Tian J, Chen L, Wei B, Shao M, Ding Y, Yin D, et al. The value of vesicant 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) in gastric malignancies. Nucl Med Commun 2004;25:825–831.
Mochiki E, Kuwano H, Katoh H, Asao T, Oriuchi N, Endo K. Evaluation of 18F-2-deoxy-2-fluoro-D-glucose positron emission tomography for gastric cancer. World J Surg 2004;28:247–253.
Wang T, Sun YE, Yu CH, Chang P, Yao SL, Tian JH, et al. Fluorine-18 fluorodeoxyglucose-positron emission tomography imaging of carcinoma of cardia or fundus of stomach (in Chinese). Zhonghua Wai Ke Za Zhi 2006;44:661–664.
Yun M, Lim JS, Noh SH, Hyung WJ, Cheong JH, Bong JK, et al. Lymph node staging of gastric cancer using (18)F-FDG PET: a comparison study with CT. J Nucl Med 2005;46:1582–1588.
Shah MA, Yeung HW, Tracola R, Ilson D, Levnor A, Capanu M, et al. The charcteristics and utility of FDG-PET/CT scans in patients with localized gastric cancer (GC). Proc Am Soc Clin Oncol 2007, Abstract 2.
Lowy AM, Feig BW, Janjan N, Rich TA, Pisters PW, Ajani JA, et al. A pilot study of preoperative chemoradiotherapy for resectable gastric cancer. Ann Surg Oncol 2001;8:519–524.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ott, K., Lordick, F., Herrmann, K. et al. The new credo: induction chemotherapy in locally advanced gastric cancer: consequences for surgical strategies. Gastric Cancer 11, 1–9 (2008). https://doi.org/10.1007/s10120-007-0448-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-007-0448-1