Abstract
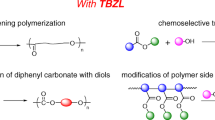
Chemoselective, living/controlled polymerizations of allyl methacrylate (AMA) and vinyl methacrylate (VMA) with/without methyl methacrylate (MMA) by using the phosphonium ylide/organoaluminum based Lewis pairs (LPs) have been realized. The P-ylide-2/AlMe(BHT)2 (P-ylide-2 = Ph3P=CHMe and BHT = 2,6-iBu2-4-MeC6H2O) was demonstrated to be superior by which homopolymers PAMAs (Mn=27.6–111.5 kg/mol and Đ=1.14–1.25) and PVMAs (Mn=28.4–78.4 kg/mol and Đ=1.12–1.18) and block copolymers PMMA-b-PAMA, PAMA-b-PVMA, PAMA-b-PMMA, PMMA-b-PAMA-b-PMMA, PAMA-b-PMMA-b-PAMA, and PAMA-b-PVMA-b-PAMA were synthesized. In the polymerizations, all of the monomers were reacted by the conjugated ester vinyl groups leaving intactly the nonconjugated acryloxy groups. The pendant acryloxy groups attached to the main chain enable further to post-functionalization by the AIBN-induced radical “thiol-ene” reaction using PhCH2SH. The thiolether side group-containing polymers PAMA-SCH2Ph and PAMA-SCH2Ph-b-PMMA-b-PAMA-SCH2Ph were thus prepared.
Similar content being viewed by others
References
Dong, Z. M.; Liu, X. H.; Liu, H. W.; Li, Y. S. Synthesis of novel star polymers with vinyl-functionalized hyperbranched core via “arm-first” strategy. Macromolecules 2010, 43, 7985–7992.
Sui, X. F.; Hempenius, M. A.; Vancso, G. Redox-active cross-linkable poly(ionic liquid)s. J. Am. Chem. Soc. 2012, 134, 4023–4025.
Barker, I. A.; El Harfi, J. E.; Adlington, K.; Howdle, S. M.; Irvine, D. J. Catalytic chain transfer mediated autopolymerization of divinylbenzene: toward facile synthesis of high alkene functional group density hyperbranched materials. Macromolecules 2012, 45, 9258–9266.
Powell, K. T.; Cheng, C.; Wooley, K. L. Complex amphiphilic hyperbranched fluoropolymers by atom transfer radical self-condensing vinyl (co)polymerization. Macromolecules 2007, 40, 4509–4515.
Stevens, D. M.; Tempelaar, S.; Dove, A. P.; Harth, E. Nanosponge formation from organocatalytically synthesized poly(carbonate) copolymers. ACS Macro Lett. 2012, 1, 915–918.
Das, A.; Theato, P. Activated ester containing polymers: opportunities and challenges for the design of functional macromolecules. Chem. Rev. 2016, 116, 1434–1495.
Bermeshev, M. V.; Chapala, P. P. Addition polymerization of functionalized norbornenes as a powerful tool for assembling molecular moieties of new polymers with versatile properties. Prog. Polym. Sci. 2018, 84, 1–46.
Zhao, Y.; Wu, H.; Zhang, Y.; Wang, X.; Yang, B.; Zhang, Q.; Ren, X.; Fu, C.; Wei, Y.; Wang, Z.; Wang, Y.; Tao, L. Postpolymerization modification of poly(dihydropyrimidin-2(1H)-thione)s via the thiourea–haloalkane reaction to prepare functional polymers. ACS Macro Lett. 2015, 4, 843–847.
Iha, R. K.; Wooley, K. L.; Nystrom, A. M.; Hawker, C. J.; Burke, D. J.; Kade, M. J.; Hawker, C. J. Applications of orthogonal “click” chemistries in the synthesis of functional soft materials. Chem. Rev. 2009, 109, 5620–5686.
Greenley, R. Z., In Polymer Handbook, 3rd ed. by Immergut, E. H., Brandup, J., Eds., Wiley, New York, 1989, p. 267
Vardareli, T. K.; Keskin, S.; Usanmaz, A. Synthesis and characterization of poly (allyl methacrylate) obtained by free radical initiator. J. Macromol. Sci., Part A: Pure Appl. Chem. 2008, 45, 302–311.
Sugiyama, F.; Satoh, K.; Kamigaito, M. Regiospecific radical polymerization of vinyl methacrylate in the presence of Lewis acids into soluble polymers with pendent vinyl ester substituents. Macromolecules 2008, 41, 3042–3048.
Ma, J.; Cheng, C.; Sun, G.; Wooley, K. L. Well-defined polymers bearing pendent alkene functionalities via selective RAFT polymerization. Macromolecules 2008, 41, 9080–9089.
Mohan, Y. M.; Raghunadh, V.; Sivaram, S.; Baskaran, D. Reactive polymers bearing styrene pendants through selective anionic polymerization of 4-vinylbenzyl methacrylate. Macromolecules 2012, 45, 3387–3393.
Tanaka, S.; Goseki, R.; Ishizone, T.; Hirao, A. Synthesis of well-defined novel reactive block polymers containing a poly(1,4l divinylbenzene) segment by living anionic polymerization. Macromolecules 2014, 47, 2333–2339.
Chen, Y.; Fuchise, K.; Narumi, A.; Kawaguchi, S.; Satoh, T.; Kakuchi, T. Core-first synthesis of three-, four-, and six-armed star-shaped poly(methyl methacrylate)s by group transfer polymerization using phosphazene base. Macromolecules 2011, 44, 9091–9098.
Sogah, D. Y.; Hertler, W. R.; Webster, O. W.; Cohen, G. M. Group transfer polymerization—polymerization of acrylic monomers. Macromolecules 1987, 20, 1473–1488.
Vidal, F.; Gowda, R. R.; Chen, E. Y. X. Chemoselective, stereospecific, and living polymerization of polar divinyl monomers by chiral zirconocenium catalysts. J. Am. Chem. Soc. 2015, 137, 9469–9480.
Xu, T.; Liu, J.; Lu, X. B. Highly active half-metallocene yttrium catalysts for living and chemoselective polymerization of allyl methacrylate. Macromolecules 2015, 48, 7428–7434.
McGraw, M. L.; Chen, E. Y. X. Lewis pair polymerization: perspective on a ten-year journey. Macromolecules 2020, 53, 6102–6122.
Zhao, W.; He, J.; Zhang, Y. Lewis pairs polymerization of polar vinyl monomers. Sci. Bull. 2019, 64, 1830–1840.
Hong, M.; Chen, J.; Chen, E. Y. X. Polymerization of polar monomers mediated by main-group Lewis acid-base pairs. Chem. Rev. 2018, 118, 10551–10616.
Wang, X.; Hong, M. Precise control of molecular weight and stereospecificity in lewis pair polymerization of semifluorinated methacrylates: mechanistic studies and stereocomplex formation. Macromolecules 2020, 53, 4659–4669.
Zhang, Z.; Wang, X.; Wang, X.; Li, Y.; Hong, M. Tris(2,4-difluorophenyl)borane/triisobutylphosphine lewis pair: a thermostable and air/moisture-tolerant organic catalyst for the living polymerization of acrylates. Macromolecules 2021, 54, 8495–8502.
Ottou, W. N.; Conde-Mendizabal, E.; Pascual, A.; Wirotius, A. L.; Bourichon, D.; Vignolle, J.; Robert, F.; Landais, Y.; Sotiropoulos, J. M.; Miqueu, K.; Taton, D. Organic Lewis pairs based on phosphine and electrophilic silane for the direct and controlled polymerization of methyl methacrylate: experimental and theoretical investigations. Macromolecules 2017, 50, 762–774.
Wang, H.; Wang, Q.; He, J.; Zhang, Y. Living polymerization of acrylamides catalysed by N-heterocyclic olefin-based Lewis pairs. Polym. Chem. 2019, 10, 3597–3603.
Knaus, M. G. M.; Giuman, M. M.; Pöthig, A.; Rieger, B. End of frustration: catalytic precision polymerization with highly interacting Lewis pairs. J. Am. Chem. Soc. 2016, 138, 7776–7781.
Bai, Y.; He, J.; Zhang, Y. Ultra-high-molecular- weight polymers produced by the immortal phosphine-based catalyst system. Angew. Chem. Int. Ed. 2018, 57, 17230–17234.
McGraw, M. L.; Chen, E. Y. X. Catalytic Lewis pair polymerization of renewable methyl crotonate to high-molecular-weight polymers. ACS Catal. 2018, 8, 9877–9887.
Clarke, R. W.; McGraw, M. L.; Gowda, R. R.; Chen, E. Y. X. Lewis pair polymerization of renewable indenone to erythro-ditactic high-Tg polymers with an upcycling avenue. Macromolecules 2020, 53, 640–648.
Wang, X.; Zhang, Y.; Hong, M. Controlled and efficient polymerization of conjugated polar alkenes by lewis pairs based on sterically hindered aryloxide-substituted alkylaluminum. Molecules 2018, 23, 442–453.
Wang, Q.; Zhao, W.; Zhang, S.; He, J.; Zhang, Y. Chen, E. Y. X. Living polymerization of conjugated polar alkenes catalyzed by N-heterocyclic olefin-based frustrated Lewis pairs. ACS Catal. 2018, 8, 3571–3578.
Zhang, Y.; Miyake, G. M.; Chen, E. Y. X. Alane-based classical and frustrated lewis pairs in polymer synthesis: rapid polymerization of MMA and naturally renewable methylene butyrolactones into high-molecular-weight polymers. Angew. Chem. Int. Ed. 2010, 49, 10158–10162.
He, J.; Zhang, Y.; Falivene, L.; Caporaso, L.; Cavallo, L.; Chen, E. Y. X. Chain propagation and termination mechanisms for polymerization of conjugated polar alkenes by [Al]-based frustrated Lewis pairs. Macromolecules 2014, 47, 7765–7774.
Jia, Y.; Wang, Y.; Ren, W.; Xu, T.; Wang, J.; Lu, X. Mechanistic aspects of initiation and deactivation in N-heterocyclic olefin mediated polymerization of acrylates with alane as activator. Macromolecules 2014, 47, 1966–1972.
Jia, Y.; Ren, W.; Liu, S.; Xu, T.; Wang, Y.; Lu, X. Controlled divinyl monomer polymerization mediated by Lewis pairs: a powerful synthetic strategy for functional polymers. ACS Macro Lett. 2014, 3, 896–899.
Gowda, R. R.; Chen, E. Y. X. Chemoselective Lewis pair polymerization of renewable multivinyl-functionalized γ-butyrolactones. Phil. Trans. R. Soc. A 2017, 375, 20170003.
Xu, P.; Wu, L.; Dong, L.; Xu, X. Chemoselective polymerization of polar divinyl monomers with rare-earth/phosphine Lewis pairs. Molecules 2018, 23, 360–369.
McGraw, M. L.; Clarke, R. W.; Chen, E. Y. X. Compounded sequence control in polymerization of one-pot mixtures of highly reactive acrylates by differentiating Lewis pairs. J. Am. Chem. Soc. 2020, 142, 5969–5973.
Song, Y.; He, J.; Zhang, Y.; Gilsdorf, R. A.; Chen, E. Y. X. Recyclable cyclic bio-based acrylic polymer via pairwise monomer enchainment by a trifunctional Lewis pair. Nat. Chem. 2022, 15, 366–376.
McGraw, M. L.; Clarke, R. W.; Chen, E. Y. X. Synchronous control of chain length/sequence/topology for precision synthesis of cyclic block copolymers from monomer mixtures. J. Am. Chem. Soc. 2021, 143, 3318–3322.
Li, C.; Zhao, W.; He, J.; Zhang, Y.; Zhang, W. Single-step expeditious synthesis of diblock copolymers with different morphologies by Lewis pair polymerization-induced self-assembly. Angew. Chem. Int. Ed. 2022, 61, e202202448.
Wan, Y.; He, J.; Zhang, Y.; Chen, E. Y. X. One-step synthesis of lignin-based triblock copolymers as high-temperature and UV-blocking thermoplastic elastomers. Angew. Chem. Int. Ed. 2022, 61, e202114946.
Zhang, P.; Zhou, H.; Lu, X. Living and chemoselective (co)polymerization of polar divinyl monomers mediated by bulky Lewis pairs. Macromolecules 2019, 52, 4520–4525.
Zhao, W.; Wang, Q.; He, J.; Zhang, Y. Chemoselective and living/controlled polymerization of polar divinyl monomers by N-heterocyclic olefin based classical and frustrated Lewis pairs. Living and chemoselective (co)polymerization of polar divinyl monomers mediated by bulky Lewis pairs. Polym. Chem. 2019, 10, 4328–4335.
Chen, Z.; Zhao, W.; Liu, C.; Jiang, L.; Fu, G.; Zhang, Y.; Zhu, H. Phosphonium ylide/organoaluminum-based Lewis pairs for the highly efficient living/controlled polymerization of alkyl (meth)acrylates. Polym. Chem. 2023, 14, 2344–2354.
Transue, W. J.; Yang, J.; Nava, M.; Sergeyev, I. V.; Barnum, T. J.; McCarthy, M. C.; Cummins, C. C. Synthetic and spectroscopic investigations enabled by modular synthesis of molecular phosphaalkyne precursors. J. Am. Chem. Soc. 2018, 140, 17985–17991.
Bestmann, H. J.; Stransky, W.; Vostrowsky, O. Darstellung Lithiumsalzfreier Ylidlosungen mit Natrium-bis-(trimethylsilyl)-amid. Chem. Ber. 1976, 109, 1694–1700.
Lee, C. H.; Lee, S. J.; Park, J. W.; Kim, K. H.; Lee, B. Y.; Oh, J. S. Preparation of Al(C6F5)3 and its use for the modification of methylalumoxane. J. Mol. Catal. A-Chem. 1998, 132, 231–239.
Stapleton, R. A.; Al-Humydi, A.; Chai, J.; Galan, B. R.; Collins, S. Sterically hindered aluminum alkyls: weakly interacting scavenging agents of use in olefin polymerization. Organometallics 2006, 25, 5083–5092.
Faingol’d, E. E.; Bravaya, N. M.; Panin, A. N.; Babkina, O. N.; Saratovskikh, S. L.; Privalov, V. I. Isobutylaluminum aryloxides as metallocene activators in homo- and copolymerization of olefins. J. Appl. Polym. Sci. 2016, 133, 43276–43284.
Usually the PAMAs herein obtained are of the rich syndiotacticity, as can be determined by the calculation of the proton intigation ratios of the Me side groups at the main chain from the 1H NMR data. See: reference 18 and Bolig, A. D.; Chen, E. Y. X. ansa-Zirconocene ester enolates: synthesis, structure, reaction with organo-Lewis acids, and application to polymerization of methacrylates. J. Am. Chem. Soc. 2004, 126, 4897–4906.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos. 21972112 and 22225104) and China Postdoctoral Science Foundation (Nos. 2022TQ0115 and 2022M711297).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare no interest conflict.
Electronic Supplementary Information
10118_2023_3039_MOESM1_ESM.pdf
Chemoselective and Living/Controlled Polymerization of Alkenyl Methacrylates by the Phosphonium Ylide/Organoaluminum-Based Lewis Pairs
Rights and permissions
About this article
Cite this article
Chen, ZK., Zhao, WC., Zhao, YL. et al. Chemoselective and Living/Controlled Polymerization of Alkenyl Methacrylates by the Phosphonium Ylide/Organoaluminum Lewis Pairs. Chin J Polym Sci 42, 159–167 (2024). https://doi.org/10.1007/s10118-023-3039-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10118-023-3039-7