Abstract
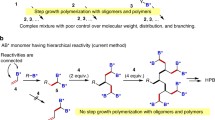
Helical poly(phenylacetylene)s (PPAs) have received extensive attention because of their features in dynamic chirality and promising applications. Therefore, understanding relationship among the polymer molecular structures, polymerization conditions and tunability of their chirality is of key scientific value. Recently, we developed a novel class of dendronized PPAs carrying 3-fold dendritic oligo(ethylene glycols) (OEGs) via alanine linkage, and found that these bulky polymers exhibited tunable helical conformations through thermally-mediated dehydration and aggregation. Herein, we report on synthesis of a homologous series of dendronized PPAs that carry 2-fold, 3-fold or 6-fold dendritic OEG pendants, and focus on effects of molecular topological structures, peripheral units and polymerization solvents on the thermoresponsiveness and their conformation switching behaviors. Effects of branching density and peripheral units (ethoxyl or methoxyl) of the dendritic OEG pendants were examined, and found to play a decisive role on the helical conformation and thermoresponsiveness of these dendronized PPAs due to their different bulkiness and overall hydrophilicity. In addition, different polymerization solvents were checked for their possible influence on the polymerization, thermoresponsive behavior and the chirality of the resulting polymers. For polymerization in selective solvents like water or methanol, the obtained dendronized PPAs exhibited weak thermal transitions, while polymerization in non-selective solvent like THF furnished PPAs with characteristic thermoresponsive behavior, indicating that solvents were involved in the process of polymerization of the dendronized macromonomers. More interestingly, different chiralities of the PPAs through polymerization in various solvents were retained, irrelevant to the purification process and solvents treatments. This work suggests that the topological structures together with polymerization solvents can modulate the thermoresponsive behavior and helical conformation of the dendronized PPAs.
Similar content being viewed by others
References
Okamoto, Y. Helical polymers for efficient enantiomer separation. Adv. Polym. Sci. 2013, 261, 391–414.
Leigh, T.; Fernandez-Trillo, P. Helical polymers for biological and medical applications. Nat. Rev. Chem. 2020, 4, 291–310.
Zhang, Y.; Deng, J. Chiral helical polymer materials derived from achiral monomers and their chiral applications. Polym. Chem. 2020, 11, 5407–5423.
Zheng, Z. G.; Lu, Y. Q.; Li, Q. Photoprogrammable mesogenic soft helical architectures: a promising avenue toward future chirooptics. Adv. Mater. 2020, 32, 1905318.
Li, S. Y.; Gao, R. T.; Chen, Z.; Liu, N.; Wu, Z. Q. Advances in circularly polarized luminescence materials based on helical polymers. J. Mater. Chem. C 2023, 11, 1242–1250.
Nakano, T.; Okamoto, Y. Synthetic helical polymers: conformation and function. Chem. Rev. 2001, 101, 4013–4038.
Liu, J. Z.; Lam, J. W. Y.; Tang, B. Z. Acetylenic polymers: syntheses, structures, and functions. Chem. Rev. 2009, 109, 5799–5867.
Scanga, R. A.; Reuther, J. F. Helical polymer self-assembly and chiral nanostructure formation. Polym. Chem. 2021, 12, 1857–1897.
Wen, T.; Wang, H. F.; Li, M. C.; Ho, R. M. Homochiral evolution in self-assembled chiral polymers and block copolymers. Acc. Chem. Res. 2017, 50, 1011–1021.
Zhang, L.; Wang, T.; Shen, Z.; Liu, M. Chiral nanoarchitectonics: towards the design, self-assembly, and function of nanoscale chiral twists and helices. Adv. Mater. 2016, 28, 1044–1059.
Bisoyi, H. K.; Li, Q. Light-directed dynamic chirality inversion in functional self-organized helical superstructures. Angew. Chem. Int. Ed. 2016, 55, 2994–3010.
Leiras, S.; Freire, F.; Quiñoá, E.; Riguera, R. Reversible assembly of enantiomeric helical polymers: from fibers to gels. Chem. Sci. 2015, 6, 246–253.
Yashima, E.; Maeda, K.; Iida, H.; Furusho, Y.; Nagai, K. Helical polymers: synthesis, structures and functions. Chem. Rev. 2009, 109, 6102–6211.
Freire, F.; Quiñoá, E.; Riguera, R. Supramolecular assemblies from poly(phenylacetylene)s. Chem. Rev. 2016, 116, 1242–1271.
Rey-Tarrio, F.; Guisan-Ceinos, S.; Cuerva, J. M.; Miguel, D.; Ribagorda, M.; Quinoa, E.; Freire, F. Photostability and dynamic helical behavior in chiral poly(phenylacetylene)s with a preferred screw-sense. Angew. Chem. Int. Ed. 2022, 61, 202207623.
Yashima, E.; Maeda, K. Chirality-responsive helical polymers. Macromolecules 2008, 41, 3–12.
Okamoto, Y.; Nakano, T. Asymmetric polymerization. Chem. Rev. 1994, 94, 349–372.
Zhang, A.; Ropero, F. R.; Zanuy, D., Alemán, C.; Meijer, E.; Schlüter, A. A rigid, chiral, dendronized polymer with a thermally stable, right-handed helical conformation. Chem. Eur. J. 2008, 14, 6924–6934.
Liu, K.; Zhang, X.; Tao, X.; Yan, J.; Kuang, G., Li, W., Zhang, A. Lysine-based dendronized polymers with preferred chirality. Polym. Chem. 2012, 3, 2708–2711.
Aoki, T.; Kaneko, T.; Maruyama, N.; Sumi, A.; Takahashi, M.; Sato, T.; Teraguchi, M. Helix-sense-selective polymerization of phenylacetylene having two hydroxy groups using a chiral catalytic system. J. Am. Chem. Soc. 2003, 125, 6346–6347.
Percec, V.; Aqad, E.; Peterca, M.; Rudick, J. G.; Lemon, L.; Ronda, J. C.; De, B. B.; Heiney, P. A.; Meijer, E. W. Steric communication of chiral information observed in dendronized polyacetylenes. J. Am. Chem. Soc. 2006, 128, 16365–16372.
Maeda, K.; Mochizuki, H.; Osato, K.; Yashima, E. Stimuli-responsive helical poly(phenylacetylene)s bearing cyclodextrin pendants that exhibit enantioselective gelation in response to chirality of a chiral amine and hierarchical super-structured helix formation. Macromolecules 2011, 44, 3217–3226.
Leiras, S.; Freire, F.; Seco, J.; Quinoa, E.; Ricardo, R. Controlled modulation of the helical sense and the elongation of poly(phenylacetylene)s by polar and donor effects. Chem. Sci. 2013, 4, 2735–2743.
Guan, X.; Wang, S.; Shi, G.; Zhang, J.; Wan, X. Thermoswitching of helical inversion of dynamic polyphenylacetylenes through cis-trans isomerization of amide pendants. Macromolecules 2021, 54, 4592–4600.
Xu, A.; Masuda, T.; Zhang, A. Stimuli-responsive polyacetylenes and dendronized poly(phenylacetylene)s. Polym. Rev. 2017, 57, 138–158.
Wamura, H.; Takeyama, Y.; Yamamoto, M.; Kurihara, H.; Morino, K.; Yashima, E. Chirality responsive helical poly(phenylacetylene) bearing L-proline pendants. Chirality 2011, 23, 35–42.
Sanda, F.; Shiotsuki, M.; Masuda, T. Controlled polymerization of phenylacetylenes using well-defined Rhodium catalysts. Macromol. Symp. 2015, 350, 67–75.
Motoshige, R.; Mawatari, Y.; Motoshige, A.; Yoshida, Y.; Sasaki, T.; Yoshimizu, H.; Suzuki, T.; Tsujita, Y.; Tabata, M. Mutual conversion between stretched and contracted helices accompanied by a drastic change in color and spatial structure of poly(phenylacetylene) prepared with a [Rh(nbd)Cl]2-amine catalyst. J. Polym. Sci., Part A: Polym. Chem. 2014, 52, 752–759.
Miyagawa, T.; Furuko, A.; Maeda, K.; Katagiri, H.; Furusho, Y.; Yashima, E. Dual memory of enantiomeric helices in a polyacetylene induced by a single enantiomer. J. Am. Chem. Soc. 2005, 127, 5018–5019.
Maeda, K.; Mochizuki, H.; Watanabe, M.; Yashima, E. Switching of macromolecular helicity of optically active poly(phenylacetylene)s bearing cyclodextrin pendants induced by various external stimuli. J. Am. Chem. Soc. 2006, 128, 7639–7650.
Li, S.; Liu, K.; Kuang, G.; Masuda, T.; Zhang, A. Thermoresponsive helical poly(phenylacetylene)s. Macromolecules 2014, 47, 3288–3296.
Suarez-Picado, E.; Quinoa, E.; Riguera, R.; Freire, F. Poly(phenylacetylene) amines: a general route to water-soluble helical polyamines. Chem. Mater. 2018, 30, 6908–6914.
Cheuk, K.; Lam, J.; Lai, L.; Tang, B. Syntheses, hydrogen-bonding interactions, tunable chain helicities, and cooperative supramolecular associations and dissociations of poly(phenylacetylene)s bearing L-valine pendants: toward the development of proteomimetic polyenes. Macromolecules 2003, 36, 9752–9762.
Okoshi, K.; Sakurai, S. I.; Ohsawa, S.; Kumaki, J.; Yashima, E. Control of main-chain stiffness of a helical poly(phenylacetylene) by switching on and off the intramolecular hydrogen bonding through macromolecular helicity inversion. Angew. Chem. Int. Ed. 2006, 45, 8173–8176.
Sakurai, S.-i.; Okoshi, K.; Kumaki, J.; Yashima, E. Two-dimensional surface chirality control by solvent-induced helicity inversion of a helical polyacetylene on graphite. J. Am. Chem. Soc. 2006, 128, 5650–5651.
Rodriguez, R.; Quinoa, E.; Riguera, R.; Freire F. Architecture of chiral poly(phenylacetylene)s: from compressed/highly dynamic to stretched/quasi-static helices. J. Am. Chem. Soc. 2016, 138, 30, 9620–9628.
Sakai, R.; Satoh, T.; Kakuchi, T. Polyacetylenes as colorimetric and fluorescent chemosensor for anions. Polym. Rev. 2017, 57, 159–174.
Bergueiro, J.; Freire, F.; Wendler, E.; Seco, J. M.; Quinoa, E.; Riguera, R. The ON/OFF switching by metal ions of the “sergeants and soldiers” chiral amplification effect on helical poly(phenylacetylene)s. Chem. Sci. 2014, 5, 2170–2176.
Cao, Y.; Ren, L.; Zhang, Y.; Lu, X.; Zhang, X.; Yan, J.; Li, W.; Masuda, T.; Zhang, A. Remarkable effects of anions on the chirality of thermoresponsive helical dendronized poly(phenylacetylene)s. Macromolecules 2021, 54, 7621–7631.
Tabata, M.; Mawatari, Y. Emerging π-conjugated stretched and contracted helices and their mutual conversions of substituted polyacetylenes prepared using an organo-rhodium catalyst. Polym. Rev. 2017, 57, 65–88.
Fukushima, T.; Kimura, H.; Tsuchihara, K. Color and chiroptical control of poly(phenylacetylene) films with chiral hydroxyl group. Macromolecules 2009, 42, 8619–8626.
Yoshida, Y.; Mawatari, Y.; Motoshige, A.; Motoshige, R.; Hiraoki, T.; Wagner, M.; Müllen, K.; Tabata, M. Accordion-like oscillation of contracted and stretched helices of polyacetylenes synchronized with the restricted rotation of side chains. J. Am. Chem. Soc. 2013, 135, 4110–4116.
Motoshige, R.; Mawatari, Y.; Motoshige, A.; Yoshida, Y.; Sasaki, T.; Yoshimizu, H.; Suzuki, T.; Tsujita, Y.; Tabata, M. “Mutual conversion between stretched and contracted helices accompanied by a drastic change in color and spatial structure of poly(phenylacetylene) prepared with a [Rh(nbd)Cl]2-amine catalyst”. J. Polym. Sci., Part A: Polym. Chem. 2014, 52, 752–759.
Wang, F.; Zhou, C.; Liu, K.; Yan, J.; Li, W.; Masuda, T.; Zhang, A. Thermoresponsive dendronized poly(phenylacetylene)s showing tunable helicity. Macromolecules 2019, 52, 8631–8642.
Kim, Y.; Shin, S.; Lee, M. Development of toroidal nanostructures by self-assembly: rational designs and applications. Acc. Chem. Res. 2013, 46, 2888–2897.
Kouwer, P. H. J.; Koepf, M.; Le Sage, V. A. A.; Jaspers, M.; van Buul, A. M.; Eksteen-Akeroyd, Z. H.; Woltinge, T.; Schwartz, E.; Kitto, H. J.; Hoogenboom1, R.; Picken, S. J.; Nolte, R. J. M.; Mendes, E.; Rowan, A. E. Responsive biomimetic networks from polyisocyanopeptide hydrogels. Nature 2013, 493, 651–655.
Hu, G. X.; Li, W.; Hu, Y. L.; Xu, A. Q.; Yan, J. T.; Liu, L. X.; Zhang, X. C.; Liu, K.; Zhang, A. Water-soluble chiral polyisocyanides showing thermoresponsive behavior. Macromolecules 2013, 46, 1124–1132.
Xu, G.; Zhang, J.; Jia, R.; Li, W.; Zhang, A. Topological effects of dendronized polymers on their thermoresponsiveness and microconfinement. Macromolecules 2022, 55, 630–642.
Li, W.; Zhang, A.; Chen, Y.; Feldman, K.; Wu, H.; Schluter, A. D. Low toxic, thermoresponsive dendrimers based on oligoethylene glycols with sharp and fully reversible phase transitions. Chem. Commun. 2008, 5948–5950.
Xu, G.; Zhang, J.; Qi, M.; Zhang, X.; Li, W.; Zhang, A. Thermoresponsive dendritic oligoethylene glycols. Phys. Chem. Chem. Phys. 2022, 24, 11848–11855.
Zhang, A.; Zhang, B.; Wachtersbach, E.; Schmidt, M.; Schluter, A. D. Efficient synthesis of high molar mass, first- to fourth-generation distributed dendronized polymers by the macromonomer approach. Chem. Eur. J. 2003, 9, 6083–6092.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos. 21971160 and 21971161) and Program for Professor of Special Appointment (No. Eastern Scholar TP2019039) at Shanghai Institutions of Higher Learning.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare no interest conflict.
Electronic Supplementary Information
10118_2023_2991_MOESM1_ESM.pdf
Synthesis of Helical Poly(phenylacetylene)s Carrying Dendritic Pendants with Varied Branching Densities Through Polymerization in Different Solvents
Rights and permissions
About this article
Cite this article
Sun, ZZ., Zhang, YN., Qiu, HY. et al. Synthesis of Helical Poly(phenylacetylene)s Carrying Dendritic Pendants with Varied Branching Densities Through Polymerization in Different Solvents. Chin J Polym Sci 41, 1543–1554 (2023). https://doi.org/10.1007/s10118-023-2991-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10118-023-2991-6