Abstract
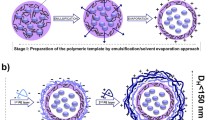
Incorporating fluorophores into polymeric nanoparticles has been testified as a feasible way to improve the emitting property and bio-compatibility of nano-emitters, which can be applied as fluorescent probes in labeling cells for imaging. Plenty of efforts have been made on the above direction. However, the size effect of nano-emitters has not been addressed yet mainly given the difficulties in controlling morphology and size of the assemblies. In our preceding study, we employed post-polymerization modification method for preparing amphiphilic copolymers, and obtained core-shell (the hydrophobic fluorophores are wrapped inside the nanoparticle to form the core) assemblies in aqueous solution. By this method, we are able to regulate the ratio of the hydrophilic/hydrophobic moieties, and thus alternate the size of the assemblies in a rather simple way. In this study, we synthesized a series of random copolymers by changing the ratio of poly(ethylene glycol) to tetraphenylethylene groups. Notably, the number of repeating units of the polymer was controlled constant for all the copolymers. The self-assembly of these copolymers resulted in different sizes of nanoparticles, and the size decreased with the decreasing fraction of poly(ethylene glycol). Interestingly, the emission of the nanoparticles showed size dependence, and smaller diameter corresponded to stronger emission. Being cultured with HeLa cells, either the large (diameter of ∼300 nm) or the small (diameter of ∼180 nm) nano-emitters allowed for very high cell viabilities up to 25 µg·mL−1. Both of them can be applied in cell imaging and provide high contrast fluorescent images.
Similar content being viewed by others
References
Jin, W. J.; Costa-Fernández, J. M.; Pereiro, R.; Sanz-Medel, A. Surface-modified CdSe quantum dots as luminescent probes for cyanide determination. Anal. Chim. Acta 2004, 522, 1–8.
Gao, X.; Yang, L.; Petros, J. A.; Marshall, F. F.; Simons, J. W.; Nie, S. In vivo molecular and cellular imaging with quantum dots. Curr. Opin. Biotechnol. 2005, 16, 63–72.
Zrazhevskiy, P.; Sena, M.; Gao, X. Designing multifunctional quantum dots for bioimaging, detection, and drug delivery. Chem. Soc. Rev. 2010, 39, 4326–4354.
Dubertret, B.; Skourides, P.; Norris, D. J.; Noireaux, V.; Brivanlou, A. H.; Libchaber, A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science 2002, 298, 1759.
Fernández-Suárez, M.; Ting, A. Y. Fluorescent probes for super-resolution imaging in living cells. Nat. Rev. Mol. Cell Biol. 2008, 9, 929–943.
Zhang, J.; Campbell, R. E.; Ting, A. Y.; Tsien, R. Y. Creating new fluorescent probes for cell biology. Nat. Rev. Mol. Cell Biol. 2002, 3, 906–918.
Iino, R.; Koyama, I.; Kusumi, A. Single molecule imaging of green fluorescent proteins in living cells: E-cadherin forms oligomers on the free cell surface. Biophys. J. 2001, 80, 2667–2677.
Nagai, T.; Ibata, K.; Park, E. S.; Kubota, M.; Mikoshiba, K.; Miyawaki, A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 2002, 20, 87–90.
Yang, Z.; Cao, J.; He, Y.; Yang, J. H.; Kim, T.; Peng, X.; Kim, J. S. Macro-/micro-environment-sensitive chemosensing and biological imaging. Chem. Soc. Rev. 2014, 43, 4563–4601.
Wu, C.; Hansen, S. J.; Hou, Q.; Yu, J.; Zeigler, M.; Jin, Y.; Burnham, D. R.; McNeill, J. D.; Olson, J. M.; Chiu, D. T. Design of highly emissive polymer dot bioconjugates for in vivo tumor targeting. Angew. Chem. Int. Ed. 2011, 123, 3492–3496.
Tao, Z.; Hong, G.; Shinji, C.; Chen, C.; Diao, S.; Antaris, A. L.; Zhang, B.; Zou, Y.; Dai, H. Biological imaging using nanoparticles of small organic molecules with fluorescence emission at wavelengths longer than 1000 nm. Angew. Chem. Int. Ed. 2013, 125, 13240–13244.
Ding, D.; Goh, C. C.; Feng, G.; Zhao, Z.; Liu, J.; Liu, R.; Tomczak, N.; Geng, J.; Tang, B. Z.; Ng, L. G., Liu, B. Ultrabright organic dots with aggregation-induced emission characteristics for real-time two-photon intravital vasculature imaging. Adv. Mater. 2013, 25, 6083–6088.
Liu, L. J.; Liu, W.; Ji, G.; Wu, Z. Y.; Xu, B.; Qian, J.; Tian, W. J. NIR emission nanoparticles based on FRET composed of AIE luminogens and NIR dyes for two-photon fluorescence imaging. Chinese J. Polym. Sci. 2019, 37, 401–408.
Zhang, X.; Zhang, X.; Wang, S.; Liu, M.; Tao, L.; Wei, Y. Surfactant modification of aggregation-induced emission material as biocompatible nanoparticles: Facile preparation and cell imaging. Nanoscale 2013, 5, 147–150.
Wu, X.; Sun, S.; Wang, Y.; Zhu, J.; Jiang, K.; Leng, Y.; Shu, Q.; Lin, H. A fluorescent carbon-dots-based mitochondria-targetable nanoprobe for peroxynitrite sensing in living cells. Biosens. Bioelectron. 2017, 90, 501–507.
Tang, L.; Wu, T.; Tang, Z. W.; Xiao, J. Y.; Zhuo, R. X.; Shi, B.; Liu, C. J. Water-soluble photoluminescent fullerene capped mesoporous silica for pH-responsive drug delivery and bioimaging. Nanotechnology 2016, 27, 315104.
Larson, D. R.; Zipfel, W. R.; Williams, R. M.; Clark, S. W.; Bruchez, M. P.; Wise, F. W.; Webb, W. W. Water-soluble quantum dots for multiphoton fluorescence imaging in vivo. Science 2003, 300, 1434–1436.
Michalet, X.; Pinaud, F. F.; Bentolila, L. A.; Tsay, J. M.; Doose, S.; Li, J. J.; Sundaresan, G.; Wu, A. M.; Gambhir, S. S.; Weiss, S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538.
Li, K.; Liu, B. Polymer-encapsulated organic nanoparticles for fluorescence and photoacoustic imaging. Chem. Soc. Rev. 2014, 43, 6570–6597.
Feng, G.; Mao, D.; Liu, J.; Goh, C. C.; Ng, L. G.; Kong, D.; Tang, B. Z.; Liu, B. Polymeric nanorods with aggregation-induced emission characteristics for enhanced cancer targeting and imaging. Nanoscale 2018, 10, 5869–5874.
Zhang, X.; Wang, K.; Liu, M.; Zhang, X.; Tao, L.; Chen, Y.; Wei, Y. Polymeric AIE-based nanoprobes for biomedical applications: Recent advances and perspectives. Nanoscale 2015, 7, 11486–11508.
Zhan, R.; Pan, Y.; Manghnani, P. N.; Liu, B. AIE polymers: Synthesis, properties, and biological applications. Macromol. Biosci. 2017, 17, 1600433.
Ma, C.; Xie, G.; Zhang, X.; Yang, L.; Li, Y.; Liu, H.; Wang, K.; Wei, Y. Biocompatible fluorescent polymers from PEGylation of an aggregation-induced emission dye. Dyes Pigments 2017, 139, 672–680.
Zhou, D.; Zhang, G.; Yu, Q.; Gan, Z. Folic acid modified polymeric micelles for intravesical instilled chemotherapy. Chinese J. Polym. Sci. 2018, 36, 479–487.
He, J.; Chen, H.; Guo, Y.; Wang, L.; Zhu, L.; Karahan, H. E.; Chen, Y. Polycondensation of a perylene bisimide derivative and L-malic acid as water-soluble conjugates for fluorescent labeling of live mammalian cells. Polymers 2018, 10, 559.
Wang, K.; Zhang, X.; Zhang, X.; Ma, C.; Li, Z.; Huang, Z.; Zhang, Q.; Wei, Y. Preparation of emissive glucose-containing polymer nanoparticles and their cell imaging applications. Polym. Chem. 2015, 6, 4455–4461.
Huang, Z.; Zhang, X.; Zhang, X.; Wang, S.; Yang, B.; Wang, K.; Yuan, J.; Tao, L.; Wei, Y. Synthesis of amphiphilic fluorescent copolymers with smart pH sensitivity via RAFT polymerization and their application in cell imaging. Polym. Bull. 2017, 74, 4525–4536.
Hua, Z.; Wilks, T. R.; Keogh, R.; Herwig, G.; Stavros, V. G.; O’Reilly, R. K. Entrapment and rigidification of adenine by a photo-cross-linked thymine network leads to fluorescent polymer nanoparticles. Chem. Mater. 2018, 30, 1408–1416.
Yang, H. M.; Park, C. W.; Park, S.; Kim, J. D. Cross-linked magnetic nanoparticles with a biocompatible amide bond for cancer-targeted dual optical/magnetic resonance imaging. Colloids Surf., B 2018, 161, 183–191.
Zhang, X.; Zhang, X.; Yang, B.; Liu, M.; Liu, W.; Chen, Y.; Wei, Y. Fabrication of aggregation induced emission dye-based fluorescent organic nanoparticles via emulsion polymerization and their cell imaging applications. Polym. Chem. 2014, 5, 399–404.
Shen, X.; Shi, Y.; Peng, B.; Li, K.; Xiang, J.; Zhang, G.; Liu, Z.; Chen, Y.; Zhang, D. Fluorescent polymeric micelles with tetraphenylethylene moieties and their application for the selective detection of glucose. Macromol. Biosci. 2012, 12, 1583–1590.
Lim, C. K.; Kim, S.; Kwon, I. C.; Ahn, C. H.; Park, S. Y. Dyecondensed biopolymeric hybrids: Chromophoric aggregation and self-assembly toward fluorescent bionanoparticles for near infrared bioimaging. Chem. Mater. 2009, 21, 5819–5825.
Lu, H.; Su, F.; Mei, Q.; Zhou, X.; Tian, Y.; Tian, W.; Johnson, R. H.; Meldrum, D. R. A series of poly[N-(2-hydroxypropyl)methacrylamide] copolymers with anthracene-derived fluorophores showing aggregation-induced emission properties for bioimaging. J. Polym. Sci., Part A: Polym. Chem. 2012, 50, 890–899.
Zhang, X.; Zhang, X.; Yang, B.; Hui, J.; Liu, M.; Chi, Z.; Liu, S.; Xu, J.; Wei, Y. Facile preparation and cell imaging applications of fluorescent organic nanoparticles that combine AIE dye and ring-opening polymerization. Polym. Chem. 2014, 5, 318–322.
Zhang, X.; Zhang, X.; Yang, B.; Hui, J.; Liu, M.; Liu, W.; Chen, Y.; Wei, Y. PEGylation and cell imaging applications of AIE based fluorescent organic nanoparticles via ring-opening reaction. Polym. Chem. 2014, 5, 689–693.
Chithrani, B. D.; Chan, W. C. W. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007, 7, 1542–1550.
Barua, S.; Yoo, J. W.; Kolhar, P.; Wakankar, A.; Gokarn, Y. R.; Mitragotri, S. Particle shape enhances specificity of antibody-displaying nanoparticles. Proc. Nat. Acad. Sci. 2013, 110, 3270.
Salata, O. V. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 2, 3.
Albanese, A.; Tang, P. S.; Chan, W. C. W. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16.
Ding, L.; Zhou, S.; Li, D.; Wu, C.; Xing, Y.; Song, B. A facile method to incorporate tetraphenylethylene into polymeric amphiphiles: High emissive nanoparticles for cell-imaging. Dyes Pigments 2019, 160, 711–716.
Gauthier, M. A.; Gibson, M. I.; Klok, H. A. Synthesis of functional polymers by post-polymerization modification. Angew. Chem. Int. Ed. 2009, 48, 48–58.
Günay, K. A.; Theato, P.; Klok, H. A. Standing on the shoulders of Hermann Staudinger: Post-polymerization modification from past to present. J. Polym. Sci., Part A: Polym. Chem. 2013, 51, 1–28.
Hong, Y.; Lam, J. W. Y.; Tang, B. Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388.
Mei, J.; Leung, N. L. C.; Kwok, R. T. K.; Lam, J. W. Y.; Tang, B. Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940.
Zhao, Y.; Wu, Y.; Yan, G.; Zhang, K. Aggregation-induced emission block copolymers based on ring-opening metathesis polymerization. RSC Adv. 2014, 4, 51194–51200.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 21674075 and 21233003), the Natural Science Foundation of Jiangsu Province (BK20161211), Key University Science Research Project of Jiangsu Province (17KJA150007), and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, XC., Zhou, SX., Ding, L. et al. Controllable Emission via Tuning the Size of Fluorescent Nano-probes Formed by Polymeric Amphiphiles. Chin J Polym Sci 37, 767–773 (2019). https://doi.org/10.1007/s10118-019-2256-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10118-019-2256-6