Abstract
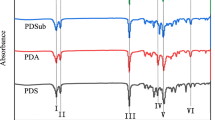
A series of new polycalixesters (PCES) were synthesized by polyesterification of calixarene dicarboxylic acid derivatives having tertiary butyl pendant groups at the upper rim using five different diols. All polyesters were readily soluble in polar solvents such as NMP (N-methylpyrrolidone), DMF (dimethylformamide), DMSO (dimethylsulfoxide), pyridine, THF (tetrahydrofurane), HMPA (hexamethylenephosphoramide) and DMAC (dimethylacetamide). The PCES were also partially soluble in TCE (tetrachloroethane) and ethanol and they were unsoluble in aceton. The glass transition temperatures of polyesters were between 80–184 °C, the crystallinity temperatures of polyesters were between 130–212 °C and the melting temperatures of polyesters were between 185–234 °C, as determined by differential scanning calorimeter (DSC). The inherent viscosities of polyesters were obtained from 0.55 dL/mg to 0.61 dL/mg. The temperatures at 10% weight loss of polyesters ranged from 182 °C to 237 °C. The temperatures at 25% weight loss of polyesters ranged from 258 °C to 331 °C. The half weight loss (50%) temperatures of polyesters were among 315 °C to 371 °C and the char yields at 600 °C were determined within 13% to 22.3% in N2 atmosphere, as determined by thermo gravimetric analysis (TGA). The polyester, PES3, has the higher melting point (234 °C) and higher inherent viscosity (molecular weight) than the other polyesters.
Similar content being viewed by others
References
Carothers, W.H., J. Am. Chem. Soc. 1929, 31: 2548
Merk, H. and Whitby, G.S., Collected papers of WH Carothers on polymerization, Interscience, New York, 1942, p. 420
Economy, J.J., Macromol. Sci. Chem., 1984, 21: 1705
Hergenrother, P., Encycl. Polym. Sci. Eng., 1988, 13: 55
Bruma, M., Sava, I., Hamciuc, E., Hamciuc, C., Belomoina, N.M. and Krongiuz, E.S., Angew. Makromol. Chem., 1992, 194: 179
Patel, P.M., Patel, S.K. and Patel, K.C., Eur. Polym. J., 2000, 36: 861
Zhang, L. and Huang, W.Y., J. Fluorine Chem., 2000, 102: 55
Gaina, C., Gaina, V. and Cozan, V., Eur. Polym. J., 2001, 37: 79
Yung, F., Bai, Y., Min, B.G., Kumar, S. and Plok, M.B., Polymer, 2003, 44: 3837
Ozden, S., Charayev, A.M. and Bazheva, R.C., J. Appl. Polym. Sci., 2008, 111: 1755
Joshi, N.B., Raja, A. and Parsania, P.H., J. Appl. Polym. Sci., 2007, 106: 2463
María, I., Bastarrachea, L. and Aguilar-Vega, M.J., J. Appl. Polym. Sci., 2006, 103: 2207
María, I., Bastarrachea, L., Vázquez-Torres, H. and Aguilar-Vega, M.J., J. Appl. Polym. Sci., 2002, 86: 2515
Shenoy, M.A., Pereira, E.A. and Parikh, P.F., J. Appl. Polym. Sci., 2005, 95: 606
Kim, S.Y., Han, S.I. and Hon, S., Polymer, 2008, 49: 3335
Stewart, F.F. and Harrup, M.K., J. Appl. Polym. Sci., 1999, 72: 1085
Femec, D.A. and Mc Caffrey, R.R., J. Appl. Poly. Sci., 1994, 52: 501
Stridsberg, K. and Albertsson, A.C., J. Polym. Sci. Part A: Polym. Chem., 2000, 38: 1774
Gutsche, C.D., Gale, P. and Ungaro, R. Calixarenes An Introduction, Royal Society of Chemistry, London, 2008, p. 276
Agrawal, Y.K., Pancholi, J.P. and Vyas, J.M., J. Sci. Ind. Res. India, 2009, 68: 745
Asfari, Z., Bohmer, W., Harrowfield, J. and Vicens, J., Eds Calixarenes, Kluwer Academic Press, Dordrecht, 2001, p. 700
Arnaud-Neu, F. and Schwing-Weill, M.J., Synth. Metals, 1997, 90: 157
Ebdelli, R., Rouis, A., Davenas, J., Bonnamour, I. and Ben Ouada, H., Sensor Lett., 2011, 9: 2241
Antipin, I., Stoikov, I.I., Pinkhassik, E.M., Fitseva, N.A., Stibor, I. and Konovalov, A.I., Tetrahedron Lett., 1997, 38: 5865
Lakouraj, M.M. and Tashakkorian, H., J. Macromol. Sci., Pure Appl. Chem., 2012, 49: 806
Gutsche, C.D., Calixarenes revisited, Stoddart, J.F., ed. The royal society of chemistry: Cambridge, London, 1998, p. 223
Sabbatini, N., Casnati, A., Fischer, C., Girardini, R., Guardigli, I., Manet, I., Sarti, G. and Ungaro, R., Inorg. Chimica Acta, 1996, 252: 19
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mighani, H., Tashakkorian, H. & Mighani, M. Synthesis and characterization of soluble polyester based on calixarene dicarboxylic acid with tertiary butyl pendant groups. Chin J Polym Sci 32, 551–557 (2014). https://doi.org/10.1007/s10118-014-1430-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10118-014-1430-0