Abstract
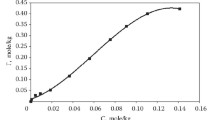
Adsorption of humic, tannic and gallic acids by a macro weakly basic ion-exchange resin JN-01 was studied. The adsorption capacity of this resin for gallic and tannic acids is much higher than that for humic acid, which can be explained on the basis of both their molecular size and ionization degree. Furthermore, humic acid is separated into different components with molecular weight in the range from 2000 Da to 100000 Da by ultra-filter, and their adsorption isotherms on resin JN-01 indicate that humic acid’s molecular weight is an important factor which makes significant influence on adsorption. Finally, changes in the amount of Cu2+ and Pb2+ adsorbed on resin JN-01 as a function of the concentration of each of these three acids were studied. A large increase in the heavy metal ions uptake is observed in the presence of humic substance, such advantages are due to the interactions between the heavy metal ions and the unbound functional groups of the adsorbed organic acids.
Similar content being viewed by others
References
Reija, E.K., Jörg, H.L. and Jaakko, A.P., Water Res., 2007, 41(12): 2715
Calace, N., Palmieri, N., Mirante, S., Petronio, B.M. and Pietroletti, M., Water Res., 2006, 40(6): 1109
Weng, L.P., Willem, H., Riemsdijk, V., Luuk, K.K. and Tjisse, H., Environ. Sci. Technol., 2006, 40(24): 7494
Chen, B.L., Elizabeth, J.J., Benny, C., Zhu, L.Z. and Xing, B.S., Environ. Sci. Technol., 2005, 39(16): 6138
Kazpard, V., Lartiges, B.S., Frochot, C., Viriot, M.L., Portal, J.M., Görner, T. and Bersillon, J.L., Water Res., 2006, 40(10): 1965
Weng, Y.H., Li, K.C., Lin Han, C.H. and Huang, C.P., Water Res., 2006, 40(9): 1783
Yu, C.H., Wu, C.H., Lin, C.H., Hsiao, C.H. and Lin, C.F., Sep. Purif. Technol., 2008, 64(2): 206
Wang, S.B., Terdkiatburana, T. and Tadé, M.O., Sep. Purif., Technol., 2008, 62(1): 64
James, E.K., Tanju, K., Walter, J. and Weber, J., Environ. Sci. Technol., 1996, 30(4): 1344
Tanju, K., James, E.K., Mark, A.S. and Walter, J.W., Environ. Sci. Technol., 1996, 30(7): 2187
Cai, Z.X., Jaeshin, K.M. and Benjamin, M., Environ. Sci. Technol., 2008, 42(2): 619
Treavor, H.B. and Philip, C.S., Environ. Sci. Technol., 2008, 42(2): 608
Lguirati, A., Baddi, G.A., Mousadik, A.E. and Ngo, H.H., Int. Biodeter. Biodegr., 2005, 56(2): 8
Allard, B., Geoderma, 2006, 130(1): 77
Steelink, C., “Implications of Elemental Characteristics of Humic Substances. Humic substances in Soil, Sediment, and Water”, Aiken G. R., New York, 1985, p.457
Gonzalez-Vila, F.J., Martin, F., Del Rio, J.C. and Kim, I.S., The Science of the Total Environment, 1992, 117(3): 335
Slejko, F.L., “Adsorption Technology: A Step-by-Step Approach to Process Evaluation and Application”, Marcel Dekker, New York, 1985, p.13
James, S., J. Colloid Interface Sci., 1969, 31(1): 116
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was financially supported by the State Key Program of National Natural Science (No. 50938004) and the National Nature Science Fund (No. 50778088) and China National Funds for Distinguished Young Scientists (No. 50825802).
Rights and permissions
About this article
Cite this article
Wang, Jn., Zhou, Y., Li, Am. et al. Adsorption of humic substances by macro weakly basic ion-exchange resin and their effects on removal of Cu2+ and Pb2+ . Chin J Polym Sci 28, 427–435 (2010). https://doi.org/10.1007/s10118-010-9050-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10118-010-9050-9