Abstract
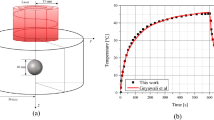
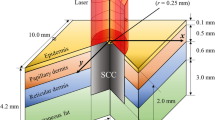
The aim of this study is to investigate how the introduction of Gold nanoparticles GNPs into a skin tumor affects the ability to absorb laser light during multicolor laser exposure. The Monte Carlo Geant4 technique was used to construct a cubic geometry simulating human skin, and a 5 mm tumor spheroid was implanted at an adjustable depth x. Our findings show that injecting a very low concentration of 0.01% GNPs into a tumor located 1 cm below the skin’s surface causes significant laser absorption of up to 25%, particularly in the 900 nm to 1200 nm range, resulting in a temperature increase of approximately 20%. It is an effective way to raise a tumor’s temperature and cause cell death while preserving healthy cells. The addition of GNPs to a tumor during polychromatic laser exposure with a wavelength ranging from 900 nm to 1200 nm increases laser absorption and thus temperature while preserving areas without GNPs.







Similar content being viewed by others
Availability of supporting data
We confirm that all data related to this article is available for accessibility whenever required.
References
Pashazadeh A, Boese A, Friebe M (2019) Radiation therapy techniques in the treatment of skin cancer: an overview of the current status and outlook. J Dermatological Treat, page 1–41
Jeynes JCG, Wordingham F, Moran LJ et al (2019) Monte Carlo simulations of heat deposition during photothermal skin cancer therapy using NP’s. Biomolecules 9(3):343
Bohara RA (2019) Introduction and types of Hybrid nanostructures for Medical Applications. Hybrid Nanostructures for Cancer Theranostics
Ash C, Dubec M, Donne K, Bashford T (2017) Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med Sci 32(8):1909–1918
Martı́nez Maestro D, del Rosal L (2014) Jaque. NP’s for photothermal therapies. Nanoscale 6(16):9494–9530
Loo C, Lowery A, Halas N, West J, Drezek R (2005) Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett 5(4):709–711
Ivan H, El-Sayed X, Huang, Mostafa A, El-Sayed (2005) Surface plasmon resonance scattering and absorption of anti-egfr antibody conjugated GNP’s. Nano Lett 5(5):829–834
Xiaohua Huang Ivan H, El-Sayed W, Qian, Mostafa A, El-Sayed (2006) Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc 128(6):2115–2120
Marcella MAURO, Matteo CROSERA, Carlotta BIANCO et al (2015) Permeation of platinum and rhodium NP’s through intact and damaged human skin. J Nanopart Res 17:1–11
Valentine RM, Ibbotson SH, Wood K, Brown CT, Moseley H (2013) Modelling fluorescence in clinical photodynamic therapy. Photochem Photobiol Sci 12(1):203–213
Campbell CL, Brown CT, Wood K, Moseley H Modelling topical photodynamic therapy treatment including the continuous production of protoporphyrin ix. Phys Med Biol., 61(21):7507–7521.,2016.
Campbell CL, Wood K, Brown CT, Moseley H (2016) Monte Carlo modelling of photodynamic therapy treatments comparing clustered three dimensional tumour structures with homogeneous tissue structures. Phys Med Biol 61(13):4840–4854
Zeng L, Gowda BHJ, Ahmed MG et al (2023) Advancements in nanoparticle-based treatment approaches for skin cancer therapy. Mol Cancer 22:10. https://doi.org/10.1186/s12943-022-01708-4
Gao Q, Zhang J, Gao J, Zhang Z, Zhu H, Wang D (2021) GNP’s in Cancer Theranostics. Front Bioeng Biotechnol 9:647905. https://doi.org/10.3389/fbioe.2021.647905
Cuplov V, Pain F, Jan S (2017) Simulation of nanoparticle-mediated near-infrared thermal therapy using gate. Biomed Opt Express 8(3):1665–1681
Kim D, Kim H (2022) Optimization of Photothermal Therapy Treatment Effect under various laser irradiation conditions. Int J Mol Sci 23(11):5928. https://doi.org/10.3390/ijms23115928PMID: 35682607; PMCID: PMC9180462
Kim D, Kim H (2023) Analysis of temperature behavior in biological tissue in photothermal therapy according to laser irradiation angle. Bioengineered 14(1):2252668. https://doi.org/10.1080/21655979.2023.2252668PMID: 37661750; PMCID: PMC10478739
Kim D, Paik J, Kim H (2023) Effect of GNP’s distribution radius on photothermal therapy efficacy. Sci Rep 13(1):12135. https://doi.org/10.1038/s41598-023-39040-6PMID: 37495612; PMCID: PMC10371995
Kanamori T, Miyazaki N, Aoki S et al (2021) Investigation of energy metabolic dynamism in hyperthermia-resistant ovarian and uterine cancer cells under heat stress. Sci Rep 11:14726. https://doi.org/10.1038/s41598-021-94031-9
Xiaoren Tang Feng Cao Weiyuan Ma at al (2020) Cancer cells resist hyperthermia due to its obstructed activation of caspase 3. Rep Practical Oncol Radiotherapy 25:323–326
Van der Zee J (2002) Heating the patient: a promising approach? Ann Oncol 13:1173–1184
Gaipl US, Datta NR, Ordonez SG et al (2016) Local hyperthermia in combined modality treatment of cancer. Crit Rev Oncol Hematol 97:200–210
Mayer A, Vaupel P (2012) Hypoxia and anemia: effects on tumor biology and treatment resistance. Transfus Med Hemother 39:302–308
Agostinelli S, Allison J, Amako K et al (2003) Geant4—a simulation toolkit. Nucl Instrum Methods Phys Res Sect A 506(3):250–303
Allison J, Amako K, Apostolakis J et al (2006) Geant4 developments and applications. IEEE Trans Nucl Sci 53(1):270–278
Wang W, Han T, Zhang X, Wu H, Yongan Shui (2007) &. Rayleigh Wave Reflection and Scattering Calculation by Source Regeneration Method. IEEE Transactions on Ultrasonics, Ferroelectrics and Frequency Control, 54(7), 1445–1453https://doi.org/10.1109/tuffc.2007.405
Gucker FT, Lin H-M (1971) A method of verifying the coefficients an and bn of the Mie theory of light scattering by nonabsorbing isotropic spheres. Journal of Colloid and Interface Science, 35(1), 139–142https://doi.org/10.1016/0021-9797(71)90194-9
Rajat Acharya (2017) Chap. 3 - Interaction of waves with medium, Editor(s): Rajat Acharya, Satellite Signal Propagation, Impairments and Mitigation, Academic Press, Pages 57–86, ISBN 9780128097328, https://doi.org/10.1016/B978-0-12-809732-8.00003-X
Setchfield K, Gorman A, Simpson AHRW, Somekh MG, Wright AJ (2023) Relevance and utility of the in-vivo and ex-vivo optical properties of the skin reported in the literature: a review [Invited]. Biomed Opt Express 14(7):3555–3583. https://doi.org/10.1364/BOE.493588
Wilhelm S et al (2016) Analysis of nanoparticle delivery to tumours. Nat Reviews Mater 1:16014
Chauhan VP et al (2012) Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat Nanotechnol 7:383–388
Polyanskiy MN (2024) Refractiveindex.info database of optical constants. Sci Data 11:94. https://doi.org/10.1038/s41597-023-02898-2
Tuersun A, Yusufu P, Yimiti T, Sidike A (2017) Refractive index sensitivity analysis of GNP’s. Optik 149:384–390
Dajun Fan Rongjie Li Minghan He (2023) Review of refractive index-matching techniques of polymethyl methacrylate in flow field visualization experiments. Int J Energy Res. https://doi.org/10.1155/2023/3413380
Aleksandar Z, Tasic Bojan D, Djordjevic Dusan K, Grozdanic, Radojkovic N (1992) Use of mixing rules in predicting refractive indexes and specific refractivities for some binary liquid mixtures. J Chem Eng Data 37(3):310–313
YAKUBOVSKY Dmitry I, STEBUNOV Yury VKIRTAEV, Roman V et al Ultrathin and ultrasmooth gold films on monolayer mos2. Advanced Materials Interfaces, Volume 2023(Article ID 3413380):1900196
Hale GM, Querry MR (1973) Optical constants of water in the 200 nm to 200 µm wavelength region. Appl Opt 12:555–563
Shrivastav RP, Sardar DK (2004) Interaction of laser radiation with biological materials: a review. Prog Quantum Electron 28(1):1–43
Venugopalan V, Vogel A (2003) Mechanisms of pulsed laser ablation of biological tissues. Chem Rev 103(2):577–644
Fan H, Chen S, Du Z, Yan T, Alimu G, Zhu L, Ma R, Alifu N, Zhang X (2022) New indocyanine green therapeutic fluorescence nanoprobes assisted high-efficient photothermal therapy for cervical cancer. Dye Pigment 200:110174
Ren Y, Qi H, Chen Q, Ruan L (2017) Thermal dosage investigation for optimal temperature distribution in gold nanoparticle enhanced photothermal therapy. Int J Heat Mass Transf 106:212–221
Lilge SL (2013) and R. P. G. Brinkmann. Laser Induced Thermal effects in Biological tissues in Encyclopedia of Biophysics G. C. K. Roberts Ed. Springer, Berlin Heidelberg, pp 1299–1305
Bucharskaya AB, Khlebtsov NG, Khlebtsov BN, Maslyakova GN, Navolokin NA, Genin VD, Genina EA, Tuchin VV (2022) Photothermal and photodynamic therapy of tumors with Plasmonic nanoparticles: challenges and prospects. Mater (Basel) 15(4):1606. https://doi.org/10.3390/ma15041606
Ashikbayeva Z, Tosi D, Balmassov D, Schena E, Saccomandi P, Inglezakis V (2019) Application of nanoparticles and nanomaterials in thermal ablation therapy of Cancer. Nanomaterials (Basel) 9(9):1195. https://doi.org/10.3390/nano9091195
D’Acunto M, Cioni P, Gabellieri E, Presciuttini G (2021) Exploiting gold nanoparticles for diagnosis and cancer treatments. Nanotechnology 32(19):192001. https://doi.org/10.1088/1361-6528/abe1ed
Yujuan Zhang Xuelin Zhan Juan Xiong (2018) Temperature-dependent cell death patterns induced by functionalized.com/scientific reports/, 8:8720(DOI:10.1038/s41598-018-26978-1):1–8
Acknowledgements
This work was supported by DGRSDT (the General Direction of Scientific Research and Technological Develop- ment, Ministry of Higher Education and Scientific research, Algeria).
Funding
This study received a financial support from Ministry of higher education and scientific research and DGRST.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Conflict of interest
The authors declare they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zerakni, F., Dib, A. & Attili, A. Enhancing tumor’s skin photothermal therapy using Gold nanoparticles : a Monte Carlo simulation. Lasers Med Sci 39, 130 (2024). https://doi.org/10.1007/s10103-024-04072-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10103-024-04072-5