Abstract
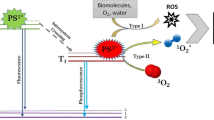
Photodynamic inactivation (PDI) technology is a promising alternative to antibiotics. This technology is defined as the inhibition of bacterial growth with photosensitizers while irradiated with low-level laser light in the wavelength of 532 ± 2.08 nm. A challenging area in this field is selecting photosensitizers with antibacterial potential. In this paper, to enhance the antibacterial efficiency, the photosensitizers (the selected plant extracts) with a high absorption peak at the selected laser frequency, 532 nm, were prepared. Low-concentration ethanolic plant extracts of Hibiscus sabdariffa and Opuntia ficus-indica were found to exhibit significant antibacterial activity against, Acinetobacter baumannii ATCC 19606 and, Staphylococcus aureus ATCC 33591 as two important human pathogenic bacteria. The effectiveness of these natural photosensitizers was measured by determining their Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) values and by performing a time-killing assay in the absence and the presence of laser irradiation. Our results showed that the combination of low-level laser irradiation and the selected photosensitizers had excellent potential for treating in vitro bacterial infections. Therefore, PDI technology has great potential as a viable alternative to traditional antibiotics for combating bacterial infections. This study presents a promising avenue for further exploration of PDI and the use of laser technology in medical science.







Similar content being viewed by others
References
Petrovska BB (2012) Historical review of medicinal plants’ usage. Pharmacogn Rev 6(11):1–5. https://doi.org/10.4103/0973-7847.95849
Yang Y, Liu Q, Shi X, Zheng Q, Chen L, Sun Y (2021) Advances in plant-derived natural products for antitumor immunotherapy. Arch Pharm Res 44(11):987–1011. https://doi.org/10.1007/s12272-021-01355-1
Gurib-Fakim A (2006) Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol Aspects of Med 27:1–93. https://doi.org/10.1016/j.mam.2005.07.008
Kolar M, Urbanek K, Latal T (2001) Antibiotic selective pressure and development of bacterial resistance. Int J Antimicrob Agents 17:357–363
Mainous AG, Diaz VA, Matheson EM, Gregorie SH, Hueston WJ (2011) Trends in hospitalizations with antibiotic-resistant infections. Public Health Rep 126:354–361
Zamani S, Nasiri MJ, Khoshgnab BN, Ashrafi A, Abdollahi A (2014) Evaluation of antimicrobial resistance pattern of nosocomial and community bacterial pathogens at a teaching hospital in tehran. Iran Acta Medica Iranica 52:182–186
Taylor PW, Stapleton PD, Luzio JP (2002) New ways to treat bacterial infections. Drug Discovery Today 7:1086–1091
Hamblin MR, Hasan T (2004) Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci 3:436–450
Quishida CCC, Mima EGDO, Jorge JH, Vergani CE, Bagnato VS, Pavarina AC (2016) Photodynamic inactivation of a multispecies biofilm using curcumin and LED light. Lasers Med Sci 31:997–1009
Mantareva V, Kussovski V, Durmuş M, Borisova E, Angelov I (2016) Photodynamic inactivation of pathogenic species Pseudomonas aeruginosa and Candida albicans with lutetium (III) acetate phthalocyanines and specific light irradiation. Lasers Med Sci 31:1591–1598
Makdoumi K, Hedin M, Bäckman A (2019) Different photodynamic effects of blue light with and without riboflavin on methicillin-resistant Staphylococcus aureus (MRSA) and human keratinocytes in vitro. Lasers Med Sci 34:1799–1805
Barroso RA, Navarro R, Tim CR, de Paula L, Ramos LD, de Oliveira Â, Araki T, Fernandes KGC, Macedo D, Assis L (2021) Antimicrobial photodynamic therapy against Propionibacterium acnes biofilms using hypericin (Hypericum perforatum) photosensitizer: in vitro study. Lasers Med Sci 36:1235–1240
Ghorbani J, Rahban D, Sh Aghamiri A, Teymouri AB (2018) Photosensitizers in antibacterial photodynamic therapy: an overview. Laser Therapy 27:293–302
Polat E, Kang K (2021) Natural Photosensitizers in Antimicrobial Photodynamic Therapy. Biomedicines 9:9060584
Huang L, El-Hussein A, Xuan W, Hamblin MR (2017) Potentiation by potassium iodide reveals that the anionic porphyrin TPPS4 is a surprisingly effective photosensitizer for antimicrobial photodynamic inactivation. J Photochem Photobiol, B 178:277–286
Huang L, Bhayana B, Xuan W, Sanchez RP, McCulloch BJ, Lalwani S, Hamblin MR (2018) Comparison of two functionalized fullerenes for antimicrobial photodynamic inactivation: Potentiation by potassium iodide and photochemical mechanisms. J Photochem Photobiol, B 186:197–206
Grinholc M, Szramka B, Kurlenda J, Graczyk A, Bielawski KP (2008) Bactericidal effect of photodynamic inactivation against methicillin-resistant and methicillin-susceptible Staphylococcus aureus is strain-dependent. J Photochem Photobiol, B 90:57–63
Niea X, Ch Jiangb Sh, Wua WC, Lva P, Wanga Q, Liua J, Ch Narha X, Caod RA, Ghiladia QW (2020) Carbon quantum dots: A bright future as photosensitizers for in vitro antibacterial photodynamic inactivation. J Photochem Photobiol, B 206:111–864
Wang Y, Guo X, Sh Zhou L, Wang YF, Xing L, Zhao Y, Zhang L, Qiu H, Zeng J, Gu Y (2021) Selective photodynamic inactivation of Helicobacter pylori by a cationic benzylidene cyclopentanone photosensitizer - an in vitro and ex vivo study. J Photochem Photobiol, B 223:112287
Regensburger J, Maisch T, Felgentrager A, Santarelli F, Baumler W (2010) A helpful technology- the luminescence detection of singlet oxygen to investigate photodynamic inactivation of bacteria (PDIB). J Biophotonics 3:5–6
Kostelanska M, Freisleben J, Hanusova ZB, Mosko T, Vik R (2019) Optimization of the photodynamic inactivation of prions by aphthalocyanine photosensitizer: the crucial involvement of singlet oxygen. J Biophotonic 12:8
Tinkler JH, Biihm F, Schalch W, Truscott TG (1994) Dietary carotenoids protect human cells from damage. J Photochem Photobiol 26:283–285
Lyu JI, Ryu J, Jin CH, Kim DG, Kim JM, Seo KS, Kim JB, Kim SH, Ahn JW, Kang SY, Kwon SJ (2020) Phenolic compounds in extracts of hibiscus acetosella (Cranberry Hibiscus) and their antioxidant and antibacterial properties. Molecules 25(18):419. https://doi.org/10.3390/molecules25184190
Khoddami A, Wilkes MA, Roberts TH (2013) Techniques for analysis of plant phenolic compounds. Molecules 18(2):2328–2375. https://doi.org/10.3390/molecules18022328
Daglia M (2011) Polyphenols as antimicrobial agents. Curr Opin Biotechnol 23:174–181
Kim J-S (2015) Production, separation and applications of phenolic-rich bio-oil–a review. Bioresour Technol 178:90–98. https://doi.org/10.1016/j.biortech.2014.08.121
Meral G, Tasar F, Kocago S, Sener C (2003) Factors affecting the antibacterial effects of Nd:YAG Laser In Vivo. Lasers Surg Med 32:197–202
Trzaska WJ, Wrigley HE, Thwaite JE, May RC (2017) Species-specific antifungal activity of blue light. Sci Rep 7:4605. https://doi.org/10.1038/s41598-017-05000-0
Posten W, Wrone DA, Dover JS, Arndt KA, Silapunt S, Alam M (2005) Low-level laser therapy for wound healing mechanism and efficacy. Am Soc Dermatol Surg 31:334–340
Dadras S, Mohajerani E, Eftekhar F, Hosseini M (2006) Different photoresponses of staphylococcus aureus and pseudomonas aeruginosa to 514, 532, and 633 nm low-level lasers in vitro. Curr Microbiol 53:282–286
Yoshida A, Sasaki H, Toyama T, Araki M, Fujioka J, Tsukiyama K, Hamada N, Yoshino F (2017) Antimicrobial effect of blue light using Porphyromonas gingivalis pigment. Scientific Reports 7:5225. https://doi.org/10.1038/s41598-017-05706-1
Brasel M, Pieranski M, Grinholc M (2020) An extended logistic model of photodynamic inactivation for various levels of irradiance using the example of Streptococcus agalactiae. Sci Rep 10:14168. https://doi.org/10.1038/s41598-020-71033-7
Mamonea L, Di Venosa G, Gándara L, Sáenz D, Vallecorsa P, Schickinger S, Rossetti MV, Batlle A, Buzzola F, Casas A (2014) Photodynamic inactivation of Gram-positive bacteria employing natural Resources. J Photochem Photobiol, B 133:80–89
Alam ST, Hwang H, Son JD, Nguyen UTT, Park J, Ch H, Kwon JK, Kang K (2021) Natural photosensitizers from Tripterygium wilfordii and their antimicrobial photodynamic therapeutic effects in a Caenorhabditis elegans model. J Photochem Photobiol, B 218:112–184
Tim M (2015) Strategies to optimize photosensitizers for photodynamic inactivation of bacteria. J Photochem Photobiol, B 150:2–10
Mishra AP, Saklani S, Sharifi-Rad M, Iriti M, Salehi B, Maurya VK, Rauf A, Milella L, Rajabi S, Baghalpour N, Sharifi-Rad (2018) Antibacterial potential of Saussurea obvallata petroleum ether extract A spiritually revered medicinal plant. J Cell Mol Biol (Noisy-le-grand) 64(8):65–70
Nocedo-Mena D, Garza-González E, González-Ferrara M, Del Rayo C-C (2020) Antibacterial activity of cissus incisa extracts against multidrug- resistant bacteria. Curr Top Med Chem 20(4):318–323. https://doi.org/10.2174/1568026619666191121123926
Benramdane E, Chougui N, Ramos PAB, Makhloufi N, Tamendjari A, Silvestre AJD, Santos SAO (2022) Lipophilic compounds and antibacterial activity of opuntia ficus-indica root extracts from algeria. Int J Mol Sci 23(19):11161. https://doi.org/10.3390/ijms231911161
Vaou N, Stavropoulou E, Voidarou C, Tsigalou C, Bezirtzoglou E (2021) Towards advances in medicinal plant antimicrobial activity: a review study on challenges and future perspectives. Microorganisms 9:2041
Da-Costa-Rocha I, Bonnlaender B, Sievers H, Pischel I, Heinrich M (2014) Hibiscus sabdariffa L. - a phytochemical and pharmacological review. Food Chem 165:424–443. https://doi.org/10.1016/j.foodchem.2014.05.002
Riaz G, Chopra R (2018) A review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomed Pharmacother 102:575–586. https://doi.org/10.1016/j.biopha.2018.03.023
Ojulari OV, Lee SG, Nam JO (2019) Beneficial Effects of Natural Bioactive Compounds from Hibiscus sabdariffa on Obesity. Molecules 24(1):210. https://doi.org/10.3390/molecules24010210
Abdel-Shafi S, Al-Mohammadi A, Sitohy M, Mosa B, Ismaiel A, Enan G, Osman A (2019) Antimicrobial activity and chemical constitution of the crude, phenolic-rich extracts of hibiscus sabdari_a. Brassica oleracea and Beta vulgaris, Molecules 24:4280
Ghareaghajlou N, Hallaj-Nezhadi S, Ghasempour Z (2021) Red cabbage anthocyanins: Stability, extraction, biological activities and applications in food systems. Food Chem 365:130482. https://doi.org/10.1016/j.foodchem.2021.130482
(2007) Final report on the safety assessment of capsicum annuum extract, capsicum annuum fruit extract, capsicum annuum resin, capsicum annuum fruit powder, capsicum frutescens fruit, capsicum frutescens fruit extract, capsicum frutescens resin, and capsaicin. Int J Toxicol 26 Suppl 1:3–106. https://doi.org/10.1080/10915810601163939
Anaya-Esparza LM, Mora ZV, Vázquez-Paulino O, Ascencio F, Villarruel-López A (2021) Bell Peppers (Capsicum annum L.) Losses andWastes: Source for Food and Pharmaceutical Applications. Molecules 26:5341
Hosseinpour-Jaghdani F, Shomali T, Gholipour-Shahraki S, Rahimi-Madiseh M, Rafieian-Kopaei M (2017) Cornus mas: a review on traditional uses and pharmacological properties. J Complement Integr Med 14(3). https://doi.org/10.1515/jcim-2016-0137
Efenberger-Szmechtyk M, Nowak A, Nowak A (2020) Cytotoxic and DNA-damaging e_ects of aronia melanocarpa, cornus mas, and chaenomeles superba leaf extracts on the human colon adenocarcinoma cell line Caco-2. Antioxidants 9:1030
Silva MA, Albuquerque TG, Pereira P, Ramalho R, Vicente F, Oliveira MBPP, Costa HS (2021) Opuntia ficus-indica (L.) Mill: a multi-benefit potential to be exploited. Molecules 26(4):951. https://doi.org/10.3390/molecules26040951
Aragona M, Lauriano ER, Pergolizzi S, Faggio C (2018) Opuntia ficus-indica (L.) Miller as a source of bioactivity compounds for health and nutrition. Nat Prod Res 32(17):2037–2049. https://doi.org/10.1080/14786419.2017.1365073
Thamkaew G, Sjöholm I, Galindo F (2021) A review of drying methods for improving the quality of dried herbs. Crit Rev Food Sci Nutr 61:11
Zhang Q-W, Lin L-G, Ye W-C (2018) Techniques for extraction and isolation of natural products: a comprehensive review. Chinese Medicine 20:13–20
Bhatta S, Janezic TS, Ratti C (2020) Review of Freeze-Drying of Plant-Based Foods. Foods 9:87
Gong D, Chen J, Li X, Sun G, Sun W (2021) A smart spectral analysis strategy-based UV and FT-IR spectroscopy fingerprint: Application to quality evaluation of compound liquorice tablets. J Pharm Biomed Anal 5(202):114172. https://doi.org/10.1016/j.jpba.2021.114172
Khuanekkaphan M, Noysang CH, Khobjai W (2020) Anti-aging potential and phytochemicals of Centella asiatica, Nelumbo nucifera, and Hibiscus sabdariffa extracts. J Adv Pharm Technol Res 11:174–178
Alsaad AJA, Mohammed LS (2021) Study of the Antioxidant Activity of Cactus (Opuntia dellienii) Fruits (Pulp and Peels) and Characterisation of their Bioactive Compounds by GC-MS. Basrah J Agric Sci 34:204–219
Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: A review. J Pharm Anal 6(2):71–79. https://doi.org/10.1016/j.jpha.2015.11.005
Subramaniam P, Reddy KRM, Eswara U (2012) Reddy Effect of different types of tea on Streptococcus mutans: an in vitro study. Indian J Dent Res 23(1):43–48
Denis TGS, Dai T, Izikson L, Astrakas CH, Anderson RR, Hamblin MR, Tegos GP (2011) All you need is light Antimicrobial photoinactivation as an evolving and emerging discovery strategy against infectious disease. Virulence 6:509–520
Williamson G (2017) The role of polyphenols in modern nutrition. Nutr Bull 42:226–235
Riaz G, Chopra R (2018) A review on phytochemistry and therapeutic uses of Hibiscus sabdari_a L. Biomed Pharmacother 102:575–586
Li P, Xu G, Li SP, Wang YT, Fan TP, Zhao QS, Zhang QW (2008) Optimizing ultra performance liquid chromatographic analysis of 10 diterpenoid compounds in Salvia miltiorrhiza using central composite design. J Agric Food Chem 56:1164–1171
Yi Y, Zhang QW, Li SL, Wang Y, Ye WC, Zhao J, Wang YT (2012) Simultaneous quantification of major flavonoids in “Bawanghua”, the edible flower of Hylocereus undatus using pressurised liquid extraction and high performance liquid chromatography. Food Chem 135:528–533
Du G, Zhao HY, Song YL, Zhang QW, Wang YT (2011) Rapid simultaneous determination of isoflavones in Radix puerariae using high-performance liquid chromatography-triple quadrupole mass spectrometry with novel shell-type column. J Sep Sci 34:2576–2585
Zeng D, Debabov D, Hartsell TL, Cano RJ, Adams S, Schuyler JA, McMillan R, Pace JL (2016) Approved glycopeptide antibacterial drugs: mechanism of action and resistance. Cold Spring Harb Perspect Med 6(12):026989. https://doi.org/10.1101/cshperspect.a026989
Holten KB, Onusko EM (2000) Appropriate prescribing of oral beta-lactam antibiotics. Am Fam Physician 62:611–620
Khardori N, Stevaux C, Ripley K (2020) Antibiotics: From the Beginning to the Future: Part 2. Indian J Pediatr 87(1):43–47. https://doi.org/10.1007/s12098-019-03113-0
Fekrazad R, Khoei F, Bahador A, Hakimiha N (2020) Comparison of different modes of photo-activated disinfection against Porphyromonas gingivalis: An in vitro study. Photodiagnosis Photodyn Ther 32:101951
Siewert B, Stuppner H (2019) The photoactivity of natural products – An overlooked potential of phytomedicines? Phytomedicine 60:152985
Wanarska E, Mielko KA, Maliszewska I, Młynarz P (2022) The oxidative stress and metabolic response of Acinetobacter baumannii for aPDT multiple photosensitization. Sci Rep 12:1913. https://doi.org/10.1038/s41598-022-05650-9
Crocker LB, Lee JH, Mital S, Mills GC, Schack S, Bistrović-Popov A, Franck ChO, Mela I, Kaminski CF, Christie G, Fruk L (2022) Tuning riboflavin derivatives for photodynamic inactivation of pathogens. Sci Rep 12:6580. https://doi.org/10.1038/s41598-022-10394-7
Astuti SD, Arifianto D, Drantantiyas NDG, Nasution AMT (2016) Efficacy of CNC-diode laser combine with chlorophylls to eliminate Staphylococcus aureus biofilm. Publishing in International Seminar on Sensors, Instrumentation, Measurement, and Metrology (ISSIMM)
Astuti SD, Suhariningsih AB, Astuti SD (2019) The efficacy of photodynamic inactivation of the diode laser in inactivation of the candida albicans biofilms with exogenous photosensitizer of papaya leaf chlorophyll. J Lasers Med Sci 10(3):215–224
Putman M, Burton R, Nahm MH (2005) Simplified method to automatically count bacterial colony forming unit. J Immunol Methods 302(1–2):99–102. https://doi.org/10.1016/j.jim.2005.05.003
Zhen J, Villani TS, Guo Y, Qi Y, Chin K, Pan M, Ch Ho JE, Simon QWu (2016) Phytochemistry, antioxidant capacity, total phenolic content and anti-inflammatory activity of Hibiscus sabdariffa leaves. Food Chem 190:673–680
Deli M, Nguimbou R, Baudelaire E, Yanou N, Scher J, Mbofung C (2020) Effect of controlled differential sieving processing on micronutrient contents and in vivo antioxidant activities of Hibiscus sabdariffa L. calyxes powder. Food Sci Biotechnol 29:1741–1753
Chauhan ES, Tiwari A (2016) A Singh Phytochemical screening of red cabbage (Brassica oleracea) powder and juice - A comparative study. Journal of Medicinal Plants Studies 4:196–199
Rybak K, Wiktor A, Witrowa-Rajchert D, Parniakov O, Nowacka M (2021) The Quality of Red Bell Pepper Subjected to Freeze-Drying Preceded by Traditional and Novel Pretreatment. Foods 10:226
Klymenko S, Kucharska AZ, Sokól-Letowska A, Piórecki N, Przybylska D, Grygorieva O (2021) Iridoids flavonoids, and antioxidant capacity of cornus mas, c officinalis, and c mas _ c officinalis fruits. Biomolecules 11:776
Silva MA, Albuquerque TG, Pereira P, Ramalho R, Vicente F, Oliveira MBPP, Costa HS (2021) Opuntia ficus-indica (L.) Mill.: A multi-benefit potential to be exploited. Molecules 26(4):951. https://doi.org/10.3390/molecules26040951
Tsao R (2010) Chemistry and biochemistry of dietary polyphenols. Nutrients 2(12):1231–1246. https://doi.org/10.3390/nu2121231
Kh Khorsandi Z, Kianmehr EG (2022) Combination effect of red light irradiation and Traychspermum ammi essential oil on colorectal cancer cells (SW480). Lasers Med Sci 37:1031–1040
Shen N, Wang T, Gan Q, Liu S, Wang L, Jin B (2022) Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem 30(383):132531. https://doi.org/10.1016/j.foodchem.2022.132531
Barbasz A, Oćwieja M, Barbasz J (2015) Cytotoxic activity of highly purified silver nanoparticles sol against cells of human immune system. Appl Biochem Biotechnol 176:817–834
Andersen M, Fossen T, Torskangerpoll K, Fossen A, Hauge U (2004) Anthocyanin from strawberry (Fragaria ananassa) with the novel aglycone, 5- carboxypyranopelargonidin. Phytochemistry 65:405–410
Kasal A, Budesinsky M, Griffiths WJ (2010) Spectroscopic Methods of Steroid Analysis. Steroid Analysis 27:161
Vinokurova NG, Vinokurova NG, Zelenkova NF, Baskunov BP (2001) Determination of diketopiperazine alkaloids of the roqefortine group by UV spectroscopy, thin-layer chromatography, and high-performance liquid chromatography. J Anal Chem 56:258–262
Maoka T (2019) Carotenoids as natural functional pigments. J Nat Med. https://doi.org/10.1007/s11418-019-01364-x
Toth M, Kukor Z, Valent S (2002) Chemical stabilization of tetrahydrobiopterin by L-ascorbic acid: contribution to placental endothelial nitric oxide synthase activity. Mol Hum Reprod 8:271–280
Galili G, Amir R, Hoefgen R, Hesse H (2005) Improving the levels of essential amino acids and sulfur metabolites in plants. Biol Chem 386(9):817–831. https://doi.org/10.1515/BC.2005.097
Author information
Authors and Affiliations
Contributions
Z. Aghaebrahimi: methodology, data collection and analysis, design, and writing the first draft of the manuscript.
M. Ranjbaran: helping to write the final manuscript, and assisting in setting up the laser setup.
J. Sabaghzadeh: methodology, design, Supervision.
S. Soudi, M. Tanhayi Ahary, S. H. Nabavi, and all authors revised and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aghaebrahimi, Z., Sabaghzadeh, J., Soudi, S. et al. Simultaneous effect of medicinal plants as natural photosensitizers and low-level laser on photodynamic inactivation. Lasers Med Sci 39, 95 (2024). https://doi.org/10.1007/s10103-024-04037-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10103-024-04037-8